Deck 7: Periodic Properties of the Elements
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/176
Play
Full screen (f)
Deck 7: Periodic Properties of the Elements
1
The effective nuclear charge of an atom is primarily affected by ________.
A)inner electrons
B)outer electrons
C)nuclear charge
D)electron distribution
E)orbital radial probability
A)inner electrons
B)outer electrons
C)nuclear charge
D)electron distribution
E)orbital radial probability
inner electrons
2
Screening of the nuclear charge by core electrons in atoms is ________.
A)less efficient than that by valence electrons
B)more efficient than that by valence electrons
C)essentially identical to that by valence electrons
D)responsible for a general decrease in atomic radius going down a group
E)both essentially identical to that by valence electrons and responsible for a general decrease in atomic radius going down a group
A)less efficient than that by valence electrons
B)more efficient than that by valence electrons
C)essentially identical to that by valence electrons
D)responsible for a general decrease in atomic radius going down a group
E)both essentially identical to that by valence electrons and responsible for a general decrease in atomic radius going down a group
more efficient than that by valence electrons
3
Of the following,which gives the correct order for atomic radius for Mg,Na,P,Si and Ar?
A)Mg > Na > P > Si > Ar
B)Ar > Si > P > Na > Mg
C)Si > P > Ar > Na > Mg
D)Na > Mg > Si > P > Ar
E)Ar > P > Si > Mg > Na
A)Mg > Na > P > Si > Ar
B)Ar > Si > P > Na > Mg
C)Si > P > Ar > Na > Mg
D)Na > Mg > Si > P > Ar
E)Ar > P > Si > Mg > Na
Na > Mg > Si > P > Ar
4
Atomic radius generally increases as we move ________.
A)down a group and from right to left across a period
B)up a group and from left to right across a period
C)down a group and from left to right across a period
D)up a group and from right to left across a period
E)down a group; the period position has no effect
A)down a group and from right to left across a period
B)up a group and from left to right across a period
C)down a group and from left to right across a period
D)up a group and from right to left across a period
E)down a group; the period position has no effect

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
5
Of the choices below,which gives the order for first ionization energies?
A)Cl > S > Al > Ar > Si
B)Ar > Cl > S > Si > Al
C)Al > Si > S > Cl > Ar
D)Cl > S > Al > Si > Ar
E)S > Si > Cl > Al > Ar
A)Cl > S > Al > Ar > Si
B)Ar > Cl > S > Si > Al
C)Al > Si > S > Cl > Ar
D)Cl > S > Al > Si > Ar
E)S > Si > Cl > Al > Ar

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is an isoelectronic series?
A)B5-, Si4-, As3-, Te2-
B)F-, Cl-, Br-, I-
C)S, Cl, Ar, K
D)Si2-, P2-, S2-, Cl2-
E)O2-, F-, Ne, Na+
A)B5-, Si4-, As3-, Te2-
B)F-, Cl-, Br-, I-
C)S, Cl, Ar, K
D)Si2-, P2-, S2-, Cl2-
E)O2-, F-, Ne, Na+

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
7
________ have the lowest first ionization energies of the groups listed.
A)Alkali metals
B)Transition elements
C)Halogens
D)Alkaline earth metals
E)Noble gases
A)Alkali metals
B)Transition elements
C)Halogens
D)Alkaline earth metals
E)Noble gases

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
8
Which compound has the smallest ionic separation?
A)NaCl
B)NaBr
C)NaI
D)NaF
E)KF
A)NaCl
B)NaBr
C)NaI
D)NaF
E)KF

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following correctly represents the third ionization of gallium?
A)Ga (g)→ Ga+ (g)+ e-
B)Ga+ (g)→ Ga2+ (g)+ e-
C)Ga2- (g)+ e- → Ga3- (g)
D)Ga2+ (g)→ Ga3+ (g)+ e-
E)Ga+ (g)+ e- → Ga2+ (g)
A)Ga (g)→ Ga+ (g)+ e-
B)Ga+ (g)→ Ga2+ (g)+ e-
C)Ga2- (g)+ e- → Ga3- (g)
D)Ga2+ (g)→ Ga3+ (g)+ e-
E)Ga+ (g)+ e- → Ga2+ (g)

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
10
The atomic radius of main-group elements generally increases down a group because ________.
A)effective nuclear charge increases down a group
B)effective nuclear charge decreases down a group
C)effective nuclear charge zigzags down a group
D)the principal quantum number of the valence orbitals increases
E)both effective nuclear charge increases down a group and the principal quantum number of the valence orbitals increases
A)effective nuclear charge increases down a group
B)effective nuclear charge decreases down a group
C)effective nuclear charge zigzags down a group
D)the principal quantum number of the valence orbitals increases
E)both effective nuclear charge increases down a group and the principal quantum number of the valence orbitals increases

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
11
Which equation correctly represents the first ionization of calcium?
A)Ca (g)→ Ca+ (g)+ e-
B)Ca (g)→ Ca- (g)+ e-
C)Ca (g)+ e- → Ca- (g)
D)Ca- (g)→ Ca (g)+ e-
E)Ca+ (g)+ e- → Ca (g)
A)Ca (g)→ Ca+ (g)+ e-
B)Ca (g)→ Ca- (g)+ e-
C)Ca (g)+ e- → Ca- (g)
D)Ca- (g)→ Ca (g)+ e-
E)Ca+ (g)+ e- → Ca (g)

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
12
Electrons in the 1s subshell are much closer to the nucleus in Ar than in He due to the larger ________ in Ar.
A)nuclear charge
B)paramagnetism
C)diamagnetism
D)Hund's rule
E)azimuthal quantum number
A)nuclear charge
B)paramagnetism
C)diamagnetism
D)Hund's rule
E)azimuthal quantum number

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
13
Rank the following in terms of decreasing first ionization energies?
A)Ne > O > N > Be > B
B)Ne > N > O > B > Be
C)Ne > O > N > B > Be
D)Ne > N > O > Be > B
E)B > Be > O > N > Ne
A)Ne > O > N > Be > B
B)Ne > N > O > B > Be
C)Ne > O > N > B > Be
D)Ne > N > O > Be > B
E)B > Be > O > N > Ne

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
14
Of the following,which gives the correct order for atomic radius for Ca,K,As,Ge and Kr?
A)Ca > K > As > Ge > Kr
B)Kr > Ge > As > K > Ca
C)Ge > As > Kr > K > Ca
D)K > Ca > Ge > As > Kr
E)Kr > As > Ge > Ca > K
A)Ca > K > As > Ge > Kr
B)Kr > Ge > As > K > Ca
C)Ge > As > Kr > K > Ca
D)K > Ca > Ge > As > Kr
E)Kr > As > Ge > Ca > K

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following correctly represents the third ionization of aluminum?
A)Al2+ (g)+ e- → Al+ (g)
B)Al (g)→ Al+ (g)+ e-
C)Al2- (g)+ e- → Al3- (g)
D)Al2+ (g)+ e- → Al3+ (g)
E)Al2+ (g)→ Al3+ (g)+ e-
A)Al2+ (g)+ e- → Al+ (g)
B)Al (g)→ Al+ (g)+ e-
C)Al2- (g)+ e- → Al3- (g)
D)Al2+ (g)+ e- → Al3+ (g)
E)Al2+ (g)→ Al3+ (g)+ e-

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
16
Atomic radius generally decreases as we move ________.
A)down a group and from right to left across a period
B)up a group and from left to right across a period
C)down a group and from left to right across a period
D)up a group and from right to left across a period
E)down a group; the period position has no effect
A)down a group and from right to left across a period
B)up a group and from left to right across a period
C)down a group and from left to right across a period
D)up a group and from right to left across a period
E)down a group; the period position has no effect

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
17
In which set of elements would all members be expected to have very similar chemical properties?
A)P, Se, I
B)Cl, Br, Na
C)Si, As, Te
D)Ne, Na, Mg
E)Br, I, At
A)P, Se, I
B)Cl, Br, Na
C)Si, As, Te
D)Ne, Na, Mg
E)Br, I, At

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
18
Which isoelectronic series is correctly arranged in order of increasing radius?
A)K+ < Ca2+ < Ar < Cl-
B)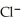 < Ar < K+ < Ca2+
< Ar < K+ < Ca2+
C)Ca2+ < Ar < K+ < Cl-
D)Ca2+ < K+ < Ar < Cl-
E)Ca2+ < K+ < Cl- < Ar
A)K+ < Ca2+ < Ar < Cl-
B)
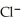 < Ar < K+ < Ca2+
< Ar < K+ < Ca2+C)Ca2+ < Ar < K+ < Cl-
D)Ca2+ < K+ < Ar < Cl-
E)Ca2+ < K+ < Cl- < Ar

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following correctly represents the first ionization of oxygen?
A)O (g)→ O+ (g)+ e-
B)O+ (g)+ e- → O2+ (g)
C)O (g)+ e- → O- (g)
D)O- (g)+ e- → O2- (g)
E)O+ (g)+ e- → O (g)
A)O (g)→ O+ (g)+ e-
B)O+ (g)+ e- → O2+ (g)
C)O (g)+ e- → O- (g)
D)O- (g)+ e- → O2- (g)
E)O+ (g)+ e- → O (g)

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
20
In which set of elements would all members be expected to have very similar chemical properties?
A)O, S, Se
B)N, O, F
C)Na, Mg, K
D)S, Se, Si
E)Ne, Na, Mg
A)O, S, Se
B)N, O, F
C)Na, Mg, K
D)S, Se, Si
E)Ne, Na, Mg

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
21
Of the following elements,________ has the most negative electron affinity.
A)S
B)Cl
C)Se
D)Br
E)I
A)S
B)Cl
C)Se
D)Br
E)I

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
22
The electron configuration of the atom with the most negative electron affinity is ________.
A)(i)
B)(ii)
C)(iii)
D)(iv)
E)(v)
A)(i)
B)(ii)
C)(iii)
D)(iv)
E)(v)

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
23
The list that correctly indicates the order of metallic character is ________.
A)B > N > C
B)F > Cl > S
C)Si > P > S
D)P > S > Se
E)Na > K > Rb
A)B > N > C
B)F > Cl > S
C)Si > P > S
D)P > S > Se
E)Na > K > Rb

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
24
Sodium is much more apt to exist as a cation than is chlorine.This is because ________.
A)chlorine is a gas and sodium is a solid
B)chlorine has a greater electron affinity than sodium does
C)chlorine is bigger than sodium
D)chlorine has a greater ionization energy than sodium does
E)chlorine is more metallic than sodium
A)chlorine is a gas and sodium is a solid
B)chlorine has a greater electron affinity than sodium does
C)chlorine is bigger than sodium
D)chlorine has a greater ionization energy than sodium does
E)chlorine is more metallic than sodium

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
25
Of the following elements,________ has the most negative electron affinity.
A)P
B)Al
C)Si
D)Cl
E)B
A)P
B)Al
C)Si
D)Cl
E)B

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
26
The electron configuration belonging to the atom with the highest second ionization energy is ________.
A)(i)
B)(ii)
C)(iii)
D)(iv)
E)(v)
A)(i)
B)(ii)
C)(iii)
D)(iv)
E)(v)

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
27
Which ion below has the largest radius?
A)Cl-
B)K+
C)Br-
D)F-
E)Na+
A)Cl-
B)K+
C)Br-
D)F-
E)Na+

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
28
Of the elements below,________ has the highest melting point.
A)Ca
B)K
C)Fe
D)Na
E)Ba
A)Ca
B)K
C)Fe
D)Na
E)Ba

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following species has the smallest ionic radius?
A)Al3+
B)Na+
C)Mg2+
D)S2-
E)Cl-
A)Al3+
B)Na+
C)Mg2+
D)S2-
E)Cl-

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following correctly represents the electron affinity of phosphorus?
A)P (g)→ P+ (g)+ e-
B)P (g)+ e- → P- (g)
C)P4 (g)+ e- → P- (g)
D)P4 (g)+ 4e- → 4P- (g)
E)P+ (g)+ e- → P (g)
A)P (g)→ P+ (g)+ e-
B)P (g)+ e- → P- (g)
C)P4 (g)+ e- → P- (g)
D)P4 (g)+ 4e- → 4P- (g)
E)P+ (g)+ e- → P (g)

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
31
The electron configuration that belongs to the atom with the lowest first ionization energy is ________.
A)(i)
B)(ii)
C)(iii)
D)(iv)
E)(v)
A)(i)
B)(ii)
C)(iii)
D)(iv)
E)(v)

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following correctly represents the electron affinity of bromine?
A)Br (g)→ Br+ (g)+ e-
B)Br (g)+ e- → Br- (g)
C)Br2 (g)+ e- → Br- (g)
D)Br2 (g)+ 2e- → 2Br- (g)
E)Br+ (g)+ e- → Br (g)
A)Br (g)→ Br+ (g)+ e-
B)Br (g)+ e- → Br- (g)
C)Br2 (g)+ e- → Br- (g)
D)Br2 (g)+ 2e- → 2Br- (g)
E)Br+ (g)+ e- → Br (g)

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
33
In the generation of most anions,the energy change (kJ/mol)that ________ an electron is ________.
A)removes, positive
B)adds, positive
C)removes, negative
D)adds, negative
E)None of the above is correct.
A)removes, positive
B)adds, positive
C)removes, negative
D)adds, negative
E)None of the above is correct.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
34
Of the following elements,________ has the most negative electron affinity.
A)O
B)K
C)B
D)Na
E)S
A)O
B)K
C)B
D)Na
E)S

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following correctly represents the second ionization of calcium?
A)Ca (g)→ Ca+ (g)+ e-
B)Ca+ (g)→ Ca2+ (g)+ e-
C)Ca- (g)+ e- → Ca2- (g)
D)Ca+ (g)+ e- → Ca2+ (g)
E)Ca+ (g)+ e- → Ca (g)
A)Ca (g)→ Ca+ (g)+ e-
B)Ca+ (g)→ Ca2+ (g)+ e-
C)Ca- (g)+ e- → Ca2- (g)
D)Ca+ (g)+ e- → Ca2+ (g)
E)Ca+ (g)+ e- → Ca (g)

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
36
The electron configuration of the atom that is expected to have the least negative electron affinity is ________.
A)(i)
B)(ii)
C)(iii)
D)(iv)
E)(v)
A)(i)
B)(ii)
C)(iii)
D)(iv)
E)(v)

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
37
Of the elements below,________ is the most metallic.
A)sodium
B)barium
C)magnesium
D)calcium
E)cesium
A)sodium
B)barium
C)magnesium
D)calcium
E)cesium

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following has the most metallic character?
A)Al
B)Ca
C)Mg
D)Sr
E)Si
A)Al
B)Ca
C)Mg
D)Sr
E)Si

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
39
Which equation correctly represents the electron affinity of calcium?
A)Ca (g)+ e- → Ca- (g)
B)Ca (g)→ Ca+ (g)+ e-
C)Ca (g)→ Ca- (g)+ e-
D)Ca- (g)→ Ca (g)+ e-
E)Ca+ (g)+ e- → Ca (g)
A)Ca (g)+ e- → Ca- (g)
B)Ca (g)→ Ca+ (g)+ e-
C)Ca (g)→ Ca- (g)+ e-
D)Ca- (g)→ Ca (g)+ e-
E)Ca+ (g)+ e- → Ca (g)

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following correctly represents the second ionization of copper?
A)Cu (g)→ Cu+ (g)+ e-
B)Cu+ (g)→ Cu2+ (g)+ e-
C)Cu- (g)+ e- → Cu2- (g)
D)Cu+ (g)+ e- → Cu2+ (g)
E)Cu+ (g)+ e- → Cu (g)
A)Cu (g)→ Cu+ (g)+ e-
B)Cu+ (g)→ Cu2+ (g)+ e-
C)Cu- (g)+ e- → Cu2- (g)
D)Cu+ (g)+ e- → Cu2+ (g)
E)Cu+ (g)+ e- → Cu (g)

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
41
Which group in the periodic table has the lowest first ionization energy?
A)VIA
B)IA
C)IIIA
D)IIA
E)VIIA
A)VIA
B)IA
C)IIIA
D)IIA
E)VIIA

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
42
Alkali metals tend to be more reactive than alkaline earth metals because ________.
A)alkali metals have lower densities
B)alkali metals have lower melting points
C)alkali metals have greater electron affinities
D)alkali metals have lower ionization energies
E)Alkali metals are not more reactive than alkaline earth metals.
A)alkali metals have lower densities
B)alkali metals have lower melting points
C)alkali metals have greater electron affinities
D)alkali metals have lower ionization energies
E)Alkali metals are not more reactive than alkaline earth metals.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
43
Reacting CO2 with water results in a(n)________ solution.
A)ionic
B)neutral
C)basic
D)acidic
E)CO2 does not react with water
A)ionic
B)neutral
C)basic
D)acidic
E)CO2 does not react with water

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following metals differ in the number of d-electrons?
A)Fe and Cu
B)Mg and Fe
C)Na and Rh
D)Cs and Ca
E)Ca and Na
A)Fe and Cu
B)Mg and Fe
C)Na and Rh
D)Cs and Ca
E)Ca and Na

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
45
Alkaline earth metals ________.
A)have the smallest atomic radius in a given period
B)form monoanions
C)form basic oxides
D)exist as triatomic molecules
E)form halides with the formula MX
A)have the smallest atomic radius in a given period
B)form monoanions
C)form basic oxides
D)exist as triatomic molecules
E)form halides with the formula MX

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
46
Which one of the following is not true about the alkali metals?
A)They are low density solids at room temperature.
B)They all readily form ions with a +1 charge.
C)They all have 2 electrons in their valence shells.
D)They are very reactive elements.
E)They have the lowest first ionization energies of the elements.
A)They are low density solids at room temperature.
B)They all readily form ions with a +1 charge.
C)They all have 2 electrons in their valence shells.
D)They are very reactive elements.
E)They have the lowest first ionization energies of the elements.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
47
The element in the periodic table that looks like a metal,is a poor thermal conductor,and acts as an electrical semiconductor is ________.
A)Sn
B)B
C)As
D)Si
E)Ge
A)Sn
B)B
C)As
D)Si
E)Ge

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following elements is the most reactive in water?
A)Na
B)Rb
C)Cs
D)Li
E)K
A)Na
B)Rb
C)Cs
D)Li
E)K

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
49
Of the following metals,________ exhibits multiple oxidation states.
A)Al
B)Rb
C)Mg
D)Ni
E)Cs
A)Al
B)Rb
C)Mg
D)Ni
E)Cs

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following oxides do not produce an acidic solution when dissolved in water?
A)SO3
B)P2O5
C)CO2
D)Al2O3
E)Cl2O
A)SO3
B)P2O5
C)CO2
D)Al2O3
E)Cl2O

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
51
The difference in metallic character is the smallest between ________.
A)Li and O
B)Rb and F
C)Na and I
D)Rb and Cl
E)Na and Mg
A)Li and O
B)Rb and F
C)Na and I
D)Rb and Cl
E)Na and Mg

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
52
All of the following reactions concerning alkali metals are correct except ________.
A)2Na (s)+ 2H2O (l)→ 2NaOH (aq)+ H2 (g)
B)Na (s)+ O2 (g)→ NaO2 (s)
C)2Na (s)+ H2 (g)→ 2NaH (s)
D)2Na (s)+ S (s)→ Na2S (s)
E)2Na (s)+ Cl2 (g)→ 2NaCl (s)
A)2Na (s)+ 2H2O (l)→ 2NaOH (aq)+ H2 (g)
B)Na (s)+ O2 (g)→ NaO2 (s)
C)2Na (s)+ H2 (g)→ 2NaH (s)
D)2Na (s)+ S (s)→ Na2S (s)
E)2Na (s)+ Cl2 (g)→ 2NaCl (s)

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
53
Consider the general valence electron configuration of ns2np5 and the following statements: (i)Elements with this electron configuration are expected to form -1 anions.
(ii)Elements with this electron configuration are expected to have large positive electron affinities.
(iii)Elements with this electron configuration are nonmetals.
(iv)Elements with this electron configuration form acidic oxides.
Which statements are true?
A)(i)and (ii)
B)(i), (ii), and (iii)
C)(ii)and (iii)
D)(i), (iii,)and (iv)
E)All statements are true.
(ii)Elements with this electron configuration are expected to have large positive electron affinities.
(iii)Elements with this electron configuration are nonmetals.
(iv)Elements with this electron configuration form acidic oxides.
Which statements are true?
A)(i)and (ii)
B)(i), (ii), and (iii)
C)(ii)and (iii)
D)(i), (iii,)and (iv)
E)All statements are true.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
54
Consider the following properties of an element: (i)It is solid at room temperature.
(ii)It easily forms an oxide when exposed to air.
(iii)When it reacts with water,hydrogen gas evolves.
(iv)It must be stored submerged in oil.
Which element fits the above description the best?
A)sulfur
B)copper
C)mercury
D)sodium
E)magnesium
(ii)It easily forms an oxide when exposed to air.
(iii)When it reacts with water,hydrogen gas evolves.
(iv)It must be stored submerged in oil.
Which element fits the above description the best?
A)sulfur
B)copper
C)mercury
D)sodium
E)magnesium

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
55
The reaction of alkali metals with oxygen produce ________.
A)oxides, peroxides, and superoxides
B)peroxides only
C)superoxides only
D)oxides only
E)none of the above
A)oxides, peroxides, and superoxides
B)peroxides only
C)superoxides only
D)oxides only
E)none of the above

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
56
The oxide of which element can react with water to give a basic solution?
A)sulfur
B)calcium
C)phosphorus
D)nitrogen
E)carbon
A)sulfur
B)calcium
C)phosphorus
D)nitrogen
E)carbon

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following is not a characteristic of metals?
A)acidic oxides
B)low ionization energies
C)malleability
D)ductility
E)These are all characteristics of metals.
A)acidic oxides
B)low ionization energies
C)malleability
D)ductility
E)These are all characteristics of metals.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the listed oxides is the most acidic?
A)SO3
B)Na2O
C)K2O
D)Al2O3
E)MgO
A)SO3
B)Na2O
C)K2O
D)Al2O3
E)MgO

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
59
Nonmetals can be ________ at room temperature.
A)solid, liquid, or gas
B)solid or liquid
C)solid only
D)liquid only
E)liquid or gas
A)solid, liquid, or gas
B)solid or liquid
C)solid only
D)liquid only
E)liquid or gas

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
60
When two elements combine to form a compound,the ________ the difference in metallic character between the two elements,the ________ the likelihood that the compound will be a ________ at room temperature.
A)smaller, greater, solid
B)greater, greater, liquid
C)greater, greater, solid
D)smaller, greater, liquid
E)greater, smaller, solid
A)smaller, greater, solid
B)greater, greater, liquid
C)greater, greater, solid
D)smaller, greater, liquid
E)greater, smaller, solid

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following statements is not true for oxygen?
A)The most stable allotrope of oxygen is O2.
B)The chemical formula of ozone is O3.
C)Dry air is about 79% oxygen.
D)Oxygen forms peroxide and superoxide anions.
E)Oxygen is a colorless gas at room temperature.
A)The most stable allotrope of oxygen is O2.
B)The chemical formula of ozone is O3.
C)Dry air is about 79% oxygen.
D)Oxygen forms peroxide and superoxide anions.
E)Oxygen is a colorless gas at room temperature.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
62
________ is a unique element and does not truly belong to any family.
A)Nitrogen
B)Radium
C)Hydrogen
D)Uranium
E)Helium
A)Nitrogen
B)Radium
C)Hydrogen
D)Uranium
E)Helium

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
63
________ is unique among the elements because its electron is not shielded from its nucleus.
A)Cesium
B)Potassium
C)Hydrogen
D)Lithium
E)Sodium
A)Cesium
B)Potassium
C)Hydrogen
D)Lithium
E)Sodium

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
64
Elements in the modern version of the periodic table are arranged in order of increasing ________.
A)oxidation number
B)atomic mass
C)average atomic mass
D)atomic number
E)number of isotopes
A)oxidation number
B)atomic mass
C)average atomic mass
D)atomic number
E)number of isotopes

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
65
The alkali metal that is naturally radioactive is ________.
A)rubidium
B)cesium
C)lithium
D)francium
E)sodium
A)rubidium
B)cesium
C)lithium
D)francium
E)sodium

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following noble gases is not reactive?
A)xenon and argon
B)helium and neon
C)xenon only
D)xenon, krypton, and argon
E)None of the above are reactive
A)xenon and argon
B)helium and neon
C)xenon only
D)xenon, krypton, and argon
E)None of the above are reactive

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
67
Hydrogen is unique among the elements because ________. 1.It is not really a member of any particular group.
2.Its electron is not at all shielded from its nucleus.
3.It is the lightest element.
4.It is the only element to exist at room temperature as a diatomic gas.
5.It exhibits some chemical properties similar to those of groups 1A and 7A.
A)1, 2, 3, 5
B)1, 2, 3, 4, 5
C)1, 4, 5
D)3, 4
E)2, 3, 4, 5
2.Its electron is not at all shielded from its nucleus.
3.It is the lightest element.
4.It is the only element to exist at room temperature as a diatomic gas.
5.It exhibits some chemical properties similar to those of groups 1A and 7A.
A)1, 2, 3, 5
B)1, 2, 3, 4, 5
C)1, 4, 5
D)3, 4
E)2, 3, 4, 5

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
68
All of the following elements can exist as allotropes except ________.
A)N
B)Ar
C)O
D)S
E)F
A)N
B)Ar
C)O
D)S
E)F

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
69
In nature,the noble gases exist as ________.
A)monatomic gaseous atoms
B)the gaseous fluorides
C)solids in rocks and in minerals
D)alkali metal salts
E)the sulfides
A)monatomic gaseous atoms
B)the gaseous fluorides
C)solids in rocks and in minerals
D)alkali metal salts
E)the sulfides

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
70
All of the halogens ________.
A)exist under ambient conditions as diatomic gases
B)tend to form positive ions of several different charges
C)tend to form negative ions of several different charges
D)exhibit metallic character
E)form salts with alkali metals with the formula MX
A)exist under ambient conditions as diatomic gases
B)tend to form positive ions of several different charges
C)tend to form negative ions of several different charges
D)exhibit metallic character
E)form salts with alkali metals with the formula MX

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
71
iodine has a(n)________ density and a(n)________ atomic radius compared to bromine.
A)smaller, greater
B)greater, smaller
C)smaller, smaller
D)greater, greater
E)equal, equal
A)smaller, greater
B)greater, smaller
C)smaller, smaller
D)greater, greater
E)equal, equal

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
72
Ozone is a a(n)________ of oxygen.
A)isotope
B)allotrope
C)precursor
D)peroxide
E)free radical
A)isotope
B)allotrope
C)precursor
D)peroxide
E)free radical

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
73
The noble gases were,until relatively recently,thought to be entirely unreactive.Experiments in the early 1960s showed that Xe could,in fact,form compounds with fluorine.The formation of compounds consisting of Xe is made possible by ________.
A)the availability of xenon atoms
B)xenon's noble gas electron configuration
C)the stability of xenon atoms
D)xenon's relatively low ionization energy
E)xenon's relatively low electron affinity
A)the availability of xenon atoms
B)xenon's noble gas electron configuration
C)the stability of xenon atoms
D)xenon's relatively low ionization energy
E)xenon's relatively low electron affinity

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
74
________ has been shown to form compounds only when it is combined with something with a tremendous ability to remove electrons from other substances.
A)Neon
B)Helium
C)Hydrogen
D)Xenon
E)Oxygen
A)Neon
B)Helium
C)Hydrogen
D)Xenon
E)Oxygen

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
75
________ is credited with developing the concept of atomic numbers.
A)Dmitri Mendeleev
B)Lothar Meyer
C)Henry Moseley
D)Ernest Rutherford
E)Michael Faraday
A)Dmitri Mendeleev
B)Lothar Meyer
C)Henry Moseley
D)Ernest Rutherford
E)Michael Faraday

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
76
Which element is solid at room temperature?
A)Cl2
B)F2
C)Br2
D)I2
E)H2
A)Cl2
B)F2
C)Br2
D)I2
E)H2

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
77
Which alkaline earth metal will not react with liquid water or with steam?
A)Be
B)Mg
C)Ca
D)Ba
E)They all react with liquid water and with steam.
A)Be
B)Mg
C)Ca
D)Ba
E)They all react with liquid water and with steam.

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
78
Lithium ion salts were originally found in Seven-Up® drinks.How many electrons does Li atom lose to become an ion?
A)4
B)3
C)0
D)1
E)2
A)4
B)3
C)0
D)1
E)2

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following elements can exist as an allotrope?
A)iron
B)carbon
C)silver
D)silicon
E)neon
A)iron
B)carbon
C)silver
D)silicon
E)neon

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck
80
Lithium ion salts were used to treat manic-depressive illness.Lithium is part of which group in the periodic table?
A)alkali metals
B)noble gases
C)alkaline earth metals
D)halogens
E)chalcogens
A)alkali metals
B)noble gases
C)alkaline earth metals
D)halogens
E)chalcogens

Unlock Deck
Unlock for access to all 176 flashcards in this deck.
Unlock Deck
k this deck


