Deck 1: An Introduction to Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/56
Play
Full screen (f)
Deck 1: An Introduction to Chemistry
1
Which is a pure substance?
A)compound
B)mixture
C)solution
D)both B and C
A)compound
B)mixture
C)solution
D)both B and C
compound
2
Which has both a definite shape and a definite volume?
A)solid
B)vapor
C)gas
D)liquid
A)solid
B)vapor
C)gas
D)liquid
solid
3
Which phase of matter contains the least force of attraction between particles?
A)solid
B)gas
C)crystal
D)liquid
A)solid
B)gas
C)crystal
D)liquid
gas
4
Why study chemistry?
A)To help inform us about our world
B)To be better able to make informed decisions
C)To help us learn a technique for identifying and solving problems
D)All the above
A)To help inform us about our world
B)To be better able to make informed decisions
C)To help us learn a technique for identifying and solving problems
D)All the above

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
5
Which phase of matter contains the greatest force of attraction between particles?
A)solid
B)vapor
C)liquid
D)gas
A)solid
B)vapor
C)liquid
D)gas

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
6
In which phase of matter are the particles furthest apart?
A)gas
B)solid
C)liquid
D)crystal
A)gas
B)solid
C)liquid
D)crystal

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
7
Which phase of matter can be compressed or expanded to the greatest degree?
A)solid
B)gas
C)crystal
D)liquid
A)solid
B)gas
C)crystal
D)liquid

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
8
Which is an observation?
A)Atoms consist of protons,neutrons,and electrons.
B)All matter is composed of atoms.
C)Water is colorless.
D)Atoms can form chemical bonds by sharing electrons.
A)Atoms consist of protons,neutrons,and electrons.
B)All matter is composed of atoms.
C)Water is colorless.
D)Atoms can form chemical bonds by sharing electrons.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
9
A simple statement of natural phenomena to which no exceptions are known under given conditions is a(n)
A)theory
B)observation
C)model
D)scientific law
A)theory
B)observation
C)model
D)scientific law

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
10
The statement,"An apple a day keeps the doctor away",is best described as a(n)
A)observation
B)law
C)theory
D)hypothesis
A)observation
B)law
C)theory
D)hypothesis

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
11
Which state of matter consists of particles in regular,repeating,three dimensional geometrical patterns?
A)liquid
B)vapor
C)solid
D)gas
A)liquid
B)vapor
C)solid
D)gas

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
12
Which state of matter consists of particles held together firmly but not rigidly? These particles are held together by strong attractive forces,are in close contact with one another,
But are able to flow by one another.
A)gas
B)liquid
C)solid
D)vapor
But are able to flow by one another.
A)gas
B)liquid
C)solid
D)vapor

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
13
The science of chemistry may involve:
A)observation
B)hypothesis development
C)experimentation
D)all the above
A)observation
B)hypothesis development
C)experimentation
D)all the above

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
14
Which has neither a definite shape nor a definite volume?
A)crystal
B)solid
C)gas
D)liquid
A)crystal
B)solid
C)gas
D)liquid

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
15
A well established hypothesis is often called a(n)
A)observation
B)fact
C)theory
D)law
A)observation
B)fact
C)theory
D)law

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
16
The statement,"An atom consists of a dense nucleus surrounded by a cloud of electrons", is best described as a(n)
A)theory
B)law
C)hypothesis
D)observation
A)theory
B)law
C)hypothesis
D)observation

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
17
Which is composed of particles with enough energy to completely overcome the attractive forces between them?
A)solid
B)gas
C)liquid
D)crystal
A)solid
B)gas
C)liquid
D)crystal

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
18
Which is a scientific observation?
A)Freezing and boiling are called physical changes
B)Water freezes at zero degrees C
C)If a substance has a density of 1.00g/mL it must be water.
D)When a substance freezes its molecules lose potential energy.
A)Freezing and boiling are called physical changes
B)Water freezes at zero degrees C
C)If a substance has a density of 1.00g/mL it must be water.
D)When a substance freezes its molecules lose potential energy.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
19
Which has a definite volume but no definite shape?
A)vapor
B)gas
C)solid
D)liquid
A)vapor
B)gas
C)solid
D)liquid

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
20
A tentative explanation of certain facts that provides the basis for further experimentation is a(n)
A)observation
B)hypothesis
C)theory
D)law
A)observation
B)hypothesis
C)theory
D)law

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
21
"Energy cannot be created nor destroyed,only transformed" is an example of a:
A)law
B)theory
C)observation
D)hypothesis
A)law
B)theory
C)observation
D)hypothesis

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
22
A substance must be heterogeneous and have a fixed composition.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
23
A key feature of the scientific method is to design and perform experiments to test hypotheses.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
24
"When Jill opened the bottle containing a clear liquid,the whole room smelled likeroses." Based on this statement we can infer that:
A)The liquid is a homogeneous mixture.
B)The liquid is a compound.
C)Inside the bottle there were a liquid and a gas.
D)Opening the bottle caused the liquid to evaporate.
A)The liquid is a homogeneous mixture.
B)The liquid is a compound.
C)Inside the bottle there were a liquid and a gas.
D)Opening the bottle caused the liquid to evaporate.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
25
Chemistry has applications in all other fields of science.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
26
Chemistry is the science that deals with the composition of substances and thetransformations they undergo.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
27
Which is a solution?
A)air
B)liquid water
C)solid iron
D)soil
A)air
B)liquid water
C)solid iron
D)soil

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
28
Which is a pure substance?
A)table salt
B)bronze
C)air
D)soil
A)table salt
B)bronze
C)air
D)soil

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
29
Which is a solution?
A)element
B)compound
C)homogeneous mixture
D)heterogeneous mixture
A)element
B)compound
C)homogeneous mixture
D)heterogeneous mixture

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
30
A mixture of citric acid and water is classified as homogeneous.What can you conclude from this description?
A)citric acid and water are clear liquids
B)the relative amounts of citric acid and water may vary
C)citric acid is a solid
D)water is present in a larger quantity compared to citric acid
A)citric acid and water are clear liquids
B)the relative amounts of citric acid and water may vary
C)citric acid is a solid
D)water is present in a larger quantity compared to citric acid

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
31
Your lab instructor gives you a red solid and tells you that it is a pure substance.Which of the following statements is true given this information?
A)The solid has a fixed composition by mass.
B)The solid is a compound.
C)The solid cannot be decomposed into simpler substances.
D)The solid cannot be melted.
A)The solid has a fixed composition by mass.
B)The solid is a compound.
C)The solid cannot be decomposed into simpler substances.
D)The solid cannot be melted.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
32
Which is a pure substance existing as different phases in a heterogeneous system?
A)Salt dissolved in water.
B)Flour suspended in water.
C)Cork floating in water.
D)Ice floating in water.
A)Salt dissolved in water.
B)Flour suspended in water.
C)Cork floating in water.
D)Ice floating in water.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following diagrams represents a solution?
A)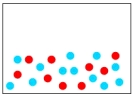
B)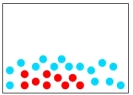
C)
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
34
" Life on Earth originated from the condensation of carbon monoxide molecules on hot mineral surfaces underground to form fatty acids,the building blocks of cellular membranes." This statement is an example of:
A)a theory
B)a hypothesis
C)a natural law
D)an observation
A)a theory
B)a hypothesis
C)a natural law
D)an observation

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
35
What can you observe in the following beaker? 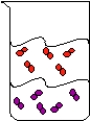
A)a pure substance in two phases.
B)a heterogeneous mixture
C)two compounds in the same phase
D)a homogeneous mixture

A)a pure substance in two phases.
B)a heterogeneous mixture
C)two compounds in the same phase
D)a homogeneous mixture

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
36
Which is a mixture?
A)copper wire
B)sugar
C)water
D)mud
A)copper wire
B)sugar
C)water
D)mud

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
37
Which is a pure substance ?
A)element
B)compound
C)mixture
D)both A and B
A)element
B)compound
C)mixture
D)both A and B

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
38
In which phase do the particles possess the greatest amount of kinetic energy?
A)solid
B)liquid
C)gas
D)crystal
A)solid
B)liquid
C)gas
D)crystal

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
39
Matter always has both mass and volume.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following diagrams represents an element?
A)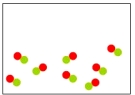
B)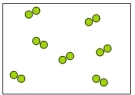
C)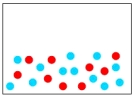
D)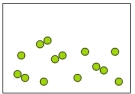
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
41
The majority of the volume of a gas is empty space.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
42
Explain how the study of chemistry can be helpful to you in your everyday life.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
43
Suppose someone brought you an unidentified material.List five questions a chemist might ask in an attempt to identify the material.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
44
Matter is classified into substances and mixtures.
Explain how these are different.
Substances are either elements or compounds.Describe what these are and give examplesof each.
Mixtures can be either heterogeneous or homogeneous.Explain how these are differentand give examples of each.
What is a solution? Give examples of solutions.
Explain how these are different.
Substances are either elements or compounds.Describe what these are and give examplesof each.
Mixtures can be either heterogeneous or homogeneous.Explain how these are differentand give examples of each.
What is a solution? Give examples of solutions.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
45
A substance may be heterogeneous or homogeneous.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
46
Air is a substance.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
47
A mixture may be heterogeneous or homogeneous.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
48
A liquid has a definite shape and a definite volume.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
49
If a piece of matter has no visible boundaries then it is a pure substance.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
50
Elements and compounds are the two types of pure substances.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
51
Explain what chemistry is.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
52
Define solid,liquid,and gas.
Explain the differences in the three phases of matter on both the macroscopic and
molecular levels.
Explain the differences in the three phases of matter on both the macroscopic and
molecular levels.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
53
Name four different ways in which you could classify or organize twenty different types of
fasteners such as nails or screws.
fasteners such as nails or screws.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
54
Mixtures can be separated by the differences in the physical properties of their
components.
What physical properties could be used to separate the components of the following mixtures?
A.Sand and salt
B.Salt and water
C.Iron filings and sulfur
D.Fine sand and coarse gravel
components.
What physical properties could be used to separate the components of the following mixtures?
A.Sand and salt
B.Salt and water
C.Iron filings and sulfur
D.Fine sand and coarse gravel

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
55
State the four steps of the scientific method.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
56
Define matter.
Explain what is meant by mass and volume.
Is everything in nature matter? If not,what else exists?
Explain what is meant by mass and volume.
Is everything in nature matter? If not,what else exists?

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck


