Deck 19: Ideal Gases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/82
Play
Full screen (f)
Deck 19: Ideal Gases
1
The specific heat of an ideal gas at constant pressure is never
A)greater than the universal gas constant.
B)less than the universal gas constant.
C)used to determine the internal energy of an ideal gas.
A)greater than the universal gas constant.
B)less than the universal gas constant.
C)used to determine the internal energy of an ideal gas.
less than the universal gas constant.
2
An ideal gas 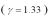 with a pressure of 1.00 atm at room temperature (300 K)is compressed from 500.0 cm3 to a final volume of 58.4 cm3.The compression is adiabatic.What final temperature of the gas?
with a pressure of 1.00 atm at room temperature (300 K)is compressed from 500.0 cm3 to a final volume of 58.4 cm3.The compression is adiabatic.What final temperature of the gas?
A)610 K
B)2600 K
C)830 K
D)300 K
E)720 K
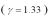 with a pressure of 1.00 atm at room temperature (300 K)is compressed from 500.0 cm3 to a final volume of 58.4 cm3.The compression is adiabatic.What final temperature of the gas?
with a pressure of 1.00 atm at room temperature (300 K)is compressed from 500.0 cm3 to a final volume of 58.4 cm3.The compression is adiabatic.What final temperature of the gas?A)610 K
B)2600 K
C)830 K
D)300 K
E)720 K
610 K
3
An ideal gas 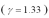 with a pressure of 1.00 atm at room temperature (300 K)is compressed from 500.0 cm3 to a final volume of 58.4 cm3.The compression is adiabatic.What final pressure of the gas?
with a pressure of 1.00 atm at room temperature (300 K)is compressed from 500.0 cm3 to a final volume of 58.4 cm3.The compression is adiabatic.What final pressure of the gas?
A)21.0 atm
B)25.1 atm
C)17.4 atm
D)8.56 atm
E)13.2 atm
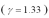 with a pressure of 1.00 atm at room temperature (300 K)is compressed from 500.0 cm3 to a final volume of 58.4 cm3.The compression is adiabatic.What final pressure of the gas?
with a pressure of 1.00 atm at room temperature (300 K)is compressed from 500.0 cm3 to a final volume of 58.4 cm3.The compression is adiabatic.What final pressure of the gas?A)21.0 atm
B)25.1 atm
C)17.4 atm
D)8.56 atm
E)13.2 atm
17.4 atm
4
For an ideal gas,the temperature measured in Kelvin is proportional to
A)the average speed of the ideal gas molecules.
B)the root-mean-squared speed of the ideal gas molecules.
C)the average kinetic energy of the ideal gas molecules.
D)None are correct.
A)the average speed of the ideal gas molecules.
B)the root-mean-squared speed of the ideal gas molecules.
C)the average kinetic energy of the ideal gas molecules.
D)None are correct.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
5
A cylinder containing nitrogen gas is at room temperature (300 K).If the volume of the gas container is held constant and the gas is heated,such that 1.46 * 104 J of energy is transferred by heat to the gas;the temperature of the gas increases by 80 K.How many moles of gas are in the container?
A)7.9 mol
B)6.1 mol
C)8.8 mol
D)5.0 mol
A)7.9 mol
B)6.1 mol
C)8.8 mol
D)5.0 mol

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
6
In a period of 6.00 s,6.00 * 1023 oxygen molecules strike a wall with an area of 2.00 cm2.If the molecules move with a speed of 400.0 m/s and strike the wall head-on in elastic collisions,what is the pressure exerted on the wall? (The mass of one O2 molecule is 5.344 * 10-26 kg.)
A)28.1 kPa
B)32.3 kPa
C)21.4 kPa
D)34.3 kPa
E)47.6 kPa
A)28.1 kPa
B)32.3 kPa
C)21.4 kPa
D)34.3 kPa
E)47.6 kPa

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
7
A cylinder containing 5.00 mol of nitrogen gas is at room temperature (300 K).If the pressure of the gas container is held constant and the gas is heated,such that 2.15 * 104 J of energy is transferred by heat to the gas,what is the increase in the temperature of the gas?
A)120 K
B)210 K
C)100 K
D)150 K
A)120 K
B)210 K
C)100 K
D)150 K

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
8
15.0 liters of an ideal monatomic gas at a pressure of 100 * 103 Pa is expanded adiabatically (no heat transfer)until the volume is tripled.If the initial temperature of the gas was 100 K,what was it final temperature after the expansion?
A)48.1 K
B)75.6 K
C)101 K
D)157 K
E)208 K
A)48.1 K
B)75.6 K
C)101 K
D)157 K
E)208 K

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
9
A cylinder containing 5.00 mol of nitrogen gas is at room temperature (300 K).If the pressure of the gas is held constant and the gas is heated,how much energy must be transferred by heat to the gas to increase its temperature by 100 K?
A)1.04 * 104 J
B)1.46 * 104 J
C)2.15 * 104 J
D)3.24 * 104 J
A)1.04 * 104 J
B)1.46 * 104 J
C)2.15 * 104 J
D)3.24 * 104 J

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
10
For an ideal gas,the temperature measured in degrees Celsius is proportional to
A)the average speed of the ideal gas molecules.
B)the root-mean-squared speed of the ideal gas molecules.
C)the average kinetic energy of the ideal gas molecules.
D)None are correct.
A)the average speed of the ideal gas molecules.
B)the root-mean-squared speed of the ideal gas molecules.
C)the average kinetic energy of the ideal gas molecules.
D)None are correct.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
11
An ideal gas is initially at a temperature of 300 K.Its volume triples while its pressure decreases by a factor of two.What is its final temperature?
A)50.0 K
B)200 K
C)450 K
D)1800 K
A)50.0 K
B)200 K
C)450 K
D)1800 K

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
12
A cylinder containing nitrogen gas is at room temperature (300 K).If the pressure of the gas container is held constant and the gas is heated,such that 2.15 * 104 J of energy is transferred by heat to the gas;the temperature of the gas increases by 80 K.How many moles of gas are in the container?
A)15 mol
B)9.2 mol
C)5.0 mol
D)13 mol
A)15 mol
B)9.2 mol
C)5.0 mol
D)13 mol

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
13
Two moles of an ideal gas are held at a constant volume of 3 liters.The change in pressure if the temperature increases by 150 C is
A)1 atm.
B)2 atm.
C)4 atm.
D)6 atm.
E)8 atm.
A)1 atm.
B)2 atm.
C)4 atm.
D)6 atm.
E)8 atm.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
14
When is the root-mean-squared speed of a collection of molecules equal to its average speed?
A)always
B)never
C)very rarely
A)always
B)never
C)very rarely

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
15
An ideal gas 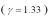 with a pressure of 1.00 atm at room temperature (300 K)is compressed from 500.0 cm3 to a final volume.The compression is adiabatic and the final pressure of the gas is 25.1 atm.What final volume of the gas?
with a pressure of 1.00 atm at room temperature (300 K)is compressed from 500.0 cm3 to a final volume.The compression is adiabatic and the final pressure of the gas is 25.1 atm.What final volume of the gas?
A)25.2 cm3
B)31.5 cm3
C)44.3 cm3
D)52.1 cm3
E)58.4 cm3
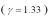 with a pressure of 1.00 atm at room temperature (300 K)is compressed from 500.0 cm3 to a final volume.The compression is adiabatic and the final pressure of the gas is 25.1 atm.What final volume of the gas?
with a pressure of 1.00 atm at room temperature (300 K)is compressed from 500.0 cm3 to a final volume.The compression is adiabatic and the final pressure of the gas is 25.1 atm.What final volume of the gas?A)25.2 cm3
B)31.5 cm3
C)44.3 cm3
D)52.1 cm3
E)58.4 cm3

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
16
A cylinder containing 5.00 mol of nitrogen gas is at room temperature (300 K).If the volume of the gas container is held constant and the gas is heated,how much energy must be transferred by heat to the gas to increase its temperature by 100 K?
A)1.04 * 104 J
B)1.46 * 104 J
C)2.15 * 104 J
D)3.24 * 104 J
A)1.04 * 104 J
B)1.46 * 104 J
C)2.15 * 104 J
D)3.24 * 104 J

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
17
Three moles of an ideal gas are held at a constant volume of 4 liters.The change in pressure if the temperature increases by 100 C is
A)1 atm.
B)2 atm.
C)4 atm.
D)6 atm.
E)8 atm.
A)1 atm.
B)2 atm.
C)4 atm.
D)6 atm.
E)8 atm.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
18
If 0.100 mol of an ideal monatomic gas has a pressure of 1.00 atm at 273 K and is cooled at constant pressure until its volume is one third its original volume.What is the amount of work done by the gas?
A)-45.2 J
B)-75.7 J
C)-100 J
D)-151 J
E)-194 J
A)-45.2 J
B)-75.7 J
C)-100 J
D)-151 J
E)-194 J

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
19
In a period of 6.00 s,7.00 * 1023 carbon dioxide molecules strike a wall with an area of 2.00 cm2.If the molecules move with a speed of 400.0 m/s and strike the wall head-on in elastic collisions,what is the pressure exerted on the wall? (The mass of one CO2 molecule is 7.348 * 10-26 kg.)
A)28.1 kPa
B)32.3 kPa
C)21.4 kPa
D)34.3 kPa
E)47.6 kPa
A)28.1 kPa
B)32.3 kPa
C)21.4 kPa
D)34.3 kPa
E)47.6 kPa

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
20
A cylinder containing 5.00 mol of nitrogen gas is at room temperature (300 K).If the volume of the gas container is held constant and the gas is heated,such that 1.46 * 104 J of energy is transferred by heat to the gas,what is the increase in the temperature of the nitrogen?
A)80 K
B)100 K
C)140 K
D)160 K
A)80 K
B)100 K
C)140 K
D)160 K

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
21
The temperature of a room at 10 C is raised to 30 C.What is the percent increase in the average speed of the molecules?
A)7.1 %
B)2.3 %
C)3.4 %
D)10 %
E)1.5 %
A)7.1 %
B)2.3 %
C)3.4 %
D)10 %
E)1.5 %

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
22
The higher the humidity in the air the more water vapor is present in the atmosphere.As the humidity increases,what happens to the density of air?
A)It increases.
B)It decreases.
C)It remains constant.
D)It may either increase or decrease,depending on the temperature.
A)It increases.
B)It decreases.
C)It remains constant.
D)It may either increase or decrease,depending on the temperature.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
23
A gas fills a rigid box at one atmosphere and 21 C.If we increase the temperature to 50 C,what is the pressure of the gas in the box?
A)1.67 atm
B)2.42 atm
C)3.35 atm
D)1.10 atm
E)2.72 atm
A)1.67 atm
B)2.42 atm
C)3.35 atm
D)1.10 atm
E)2.72 atm

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following gases has the highest rms velocity?
A)nitrogen molecules at 1 atm and 30 C
B)argon atoms at 1atm and 30 C
C)argon atoms at 2 atm and 30 C
D)oxygen molecules at 2 atm and 30 C
E)nitrogen molecules at 2 atm and 15 C
A)nitrogen molecules at 1 atm and 30 C
B)argon atoms at 1atm and 30 C
C)argon atoms at 2 atm and 30 C
D)oxygen molecules at 2 atm and 30 C
E)nitrogen molecules at 2 atm and 15 C

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
25
A tire has a gauge pressure 300kPa at 15 C.What is the gauge pressure at 45 C? Assume that the change in volume of the tire is negligible.
A)330 kPa
B)340 kPa
C)440 kPa
D)900 kPa
E)1100 kPa
A)330 kPa
B)340 kPa
C)440 kPa
D)900 kPa
E)1100 kPa

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
26
Suppose you had two atomic gases that react to form a diatomic gas A + B AB.Suppose you perform this reaction with 1 mole each of A and B in a thermally isolated chamber so no heat is exchanged with the environment.The temperature of the final system
A)will remain the same.
B)will decrease.
C)will increase.
D)could either increase or decrease depending on information that has not been provided in this problem.
A)will remain the same.
B)will decrease.
C)will increase.
D)could either increase or decrease depending on information that has not been provided in this problem.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
27
Find the most probable speed of water molecules (m =2.99 * 10-26 kg)inside your body if the water molecules can be treated as an ideal gas.Body temperature is 98.6 F or 37.0 C.
A)130 m/s
B)226 m/s
C)378 m/s
D)534 m/s
E)655 m/s
A)130 m/s
B)226 m/s
C)378 m/s
D)534 m/s
E)655 m/s

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
28
The temperature of a room at 21 C is raised such that the average speed of the molecules increases by 10%.What is the new temperature of the room?
A)51 C
B)25 C
C)63 C
D)83 C
E)42 C
A)51 C
B)25 C
C)63 C
D)83 C
E)42 C

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
29
What volume of nitrogen gas at 1 atm would be required to fill a 3 liter tank to 1800 psi? Assume that no temperature change occurs during the compression.
A)18 liter
B)367 liter
C)122 liter
D)425 liter
E)632 cm
A)18 liter
B)367 liter
C)122 liter
D)425 liter
E)632 cm

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
30
Suppose you have a pot filled with steam at 110 C and at a pressure of 1 atm.The pot has a 20 cm diameter and is 15 cm high.You place a heavy stone on the lid,so that the combined mass of the lid and stone is 45 kg.How hot do you need to heat the steam to lift the lid off the pot?
A)163 C
B)128 C
C)223 C
D)175 C
E)115 C
A)163 C
B)128 C
C)223 C
D)175 C
E)115 C

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
31
A diesel engine compresses the air adiabatically.If the engine compresses the air in it to 18 atmospheres find the temperature of the compressed air if the air enters the engine at 22 C.You may assume that air behaves as an ideal gas during this process.
A)400 C
B)410 C
C)5000 C
D)5300 C
E)5600 C
A)400 C
B)410 C
C)5000 C
D)5300 C
E)5600 C

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following gases have the highest average velocity?
A)diatomic oxygen at 21 C
B)diatomic nitrogen at 60 C
C)neon at 21 C
D)argon at 30 C
E)carbon dioxide at 50 C
A)diatomic oxygen at 21 C
B)diatomic nitrogen at 60 C
C)neon at 21 C
D)argon at 30 C
E)carbon dioxide at 50 C

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
33
A 20 cm diameter balloon at 1atm and 21 C is cooled until its diameter shrinks to 18 cm.What is the final temperature of the gas in the balloon?
A)19 C
B)0 C
C)21 C
D)-21 C
E)-59 C
A)19 C
B)0 C
C)21 C
D)-21 C
E)-59 C

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
34
What is the approximate energy required to heat 3 liters of air at standard temperature and pressure by 125 C? The volume is held constant.
A)125 J
B)175 J
C)225 J
D)345 J
E)365 J
A)125 J
B)175 J
C)225 J
D)345 J
E)365 J

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
35
One mole of a diatomic gas has an initial temperature of 293 K.Its internal energy is increased by 85.0 J.What is the final temperature of the diatomic gas?
A)293 K
B)294 K
C)295 K
D)296 K
E)297 K
A)293 K
B)294 K
C)295 K
D)296 K
E)297 K

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
36
An ideal gas is in a 1.00-m3 box at a gauge pressure of 210 kPa and a temperature of 295 K.It is then allowed to undergo free expansion into an adjacent 2.00-m3 box,so the total volume is now 3.00 m3.Find the new gauge pressure once a new equilibrium is reached.The pressure outside the box is standard atmospheric pressure,101.3 kPa.
A)2.47 kPa
B)54.4 kPa
C)70 kPa
D)104 kPa
E)Additional information is needed to answer this question.
A)2.47 kPa
B)54.4 kPa
C)70 kPa
D)104 kPa
E)Additional information is needed to answer this question.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
37
Find the rms speed of water molecules (m =2.99 * 10-26 kg)inside your body if the water molecules can be treated as an ideal gas.Body temperature is 98.6 F or 37.0 C.
A)130 m/s
B)226 m/s
C)378 m/s
D)534 m/s
E)655 m/s
A)130 m/s
B)226 m/s
C)378 m/s
D)534 m/s
E)655 m/s

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
38
A tank of gas consisting of 40% O2 and 60% Ar and volume of 2 m3 is moved from storage at 20 C to the outside on a sunny day.Initially the pressure gauge reads 1200 psi After several hours the pressure reads 1600 psi.What is the new temperature of the gas in the tank?
A)20 C
B)101 C
C)27 C
D)118 C
E)146 C
A)20 C
B)101 C
C)27 C
D)118 C
E)146 C

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
39
A diesel engine compresses the air adiabatically.If the engine compresses the air in it to a temperature of of 420 C and the compressed air if the air enters the engine at 22 C,find the pressure of the air when it reaches 420 C .You may assume that air behaves as an ideal gas during this process.
A)2.4 atm
B)3.0 atm
C)17 atm
D)18 atm
E)19 atm
A)2.4 atm
B)3.0 atm
C)17 atm
D)18 atm
E)19 atm

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
40
A gas fills a rigid box at one atmosphere and 21 C.If we increase the pressure to 1.4 atmospheres,what is the temperature of the gas in the box?
A)100 C
B)140 C
C)150 C
D)50 C
E)130 C
A)100 C
B)140 C
C)150 C
D)50 C
E)130 C

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
41
1-liter of gas undergoes first an isochoric process in which its pressure doubles,followed by an isothermal process until the original pressure is reached.Determine the final volume of the gas.
A)1 liter
B)2 liters
C)3 liters
D)0.5 liter
A)1 liter
B)2 liters
C)3 liters
D)0.5 liter

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
42
The diameter of an oxygen molecule is about 0.3 nm.The pressure required for a mean free path of 1mm at 300 K is
A)1.04 Pa.
B)6.5 Pa.
C)8.2 Pa.
D)10.4 Pa.
E)15.6 Pa.
A)1.04 Pa.
B)6.5 Pa.
C)8.2 Pa.
D)10.4 Pa.
E)15.6 Pa.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
43
At standard atmospheric pressure,at what temperature is the total kinetic energy of a bottle containing 0.15 mol of neon equal to that of a major league pitcher's fastball (0.145 kg ball travelling at 95 mph)?
A)11 K
B)70 K
C)100 K
D)The mass of neon is needed.
A)11 K
B)70 K
C)100 K
D)The mass of neon is needed.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
44
A gas expands at constant pressure from 3 liters at 15 C until the volume is 4 liters.What is the final temperature of the gas?
A)111 C
B)15 C
C)20 C
D)11 C
A)111 C
B)15 C
C)20 C
D)11 C

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
45
A cylinder with a piston contains 2.00 liters of an ideal monatomic gas at 1.00 atm and 275 K.The gas is subject to an adiabatic compression and its pressure increases to four times its initial pressure.What is the volume after the compression?
A)0.50 liters
B)0.95 liters
C)0.87 liters
D)0.46 liters
A)0.50 liters
B)0.95 liters
C)0.87 liters
D)0.46 liters

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
46
The specific heat of steam (M = 18 g/mol)at constant volume is 2.5 kJ/kg K.Given R = 8.31 J/mol .K,the specific heat at constant pressure is
A)2.96 kJ/kg.K.
B)3.51 kJ/kg.K.
C)4.98 kJ/kg.K.
D)10.81 kJ/kg.K.
E)14.98 kJ/kg.K.
A)2.96 kJ/kg.K.
B)3.51 kJ/kg.K.
C)4.98 kJ/kg.K.
D)10.81 kJ/kg.K.
E)14.98 kJ/kg.K.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
47
At standard atmospheric pressure,at what temperature is the rms speed of Krypton atoms (83.8g/mol)equal to the speed of a cruising 747 (555 mph)?
A)103 K
B)207 K
C)310 K
D)1040 K
A)103 K
B)207 K
C)310 K
D)1040 K

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
48
Uranium has two isotopes U238 and U235.Enriched uranium,with a greater fraction of U235 required by nuclear reactors,is synthesized by first forming the compound UF6 in the gaseous form and then allowing it to pass through a porous medium.The two isotopes are then separated by diffusion which depends on the rms speeds of the molecules.Given the mass of a fluorine atom is 19 amu,masses of U235 and U238 atoms are 235.043924 amu and 238.050785 amu,respectively,the ratio of the rms speeds of the two uranium hexafluoride molecules is
A)1.1000.
B)1.0043.
C)1.0420.
D)1.0680.
E)10.459.
A)1.1000.
B)1.0043.
C)1.0420.
D)1.0680.
E)10.459.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
49
A gas expands at constant pressure from 3 liters at 15 C until the temperature is 151 C.What is the final volume of the gas?
A)5.2 liters
B)4.4 liters
C)4.8 liters
D)4.0 liters
A)5.2 liters
B)4.4 liters
C)4.8 liters
D)4.0 liters

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
50
Two containers contain the same gas at different temperatures and pressures as detailed in the drawing below.The small container has a volume of 1 liter and the large container has a volume of 2 liters.The two containers are then connected to each other using a thin tube and the system is allowed to reach equal pressure and temperature in both containers.If the final pressure is 200 kPa,what is the final temperature? You may assume that the connecting tube has negligible volume and mass. 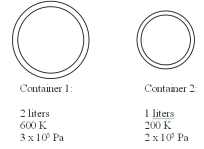
A)400 K
B)500 K
C)550 K
D)300 K

A)400 K
B)500 K
C)550 K
D)300 K

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
51
Helium gas is filled in a balloon of diameter 30 cm at 20 C and 1 atm pressure.The number of atoms of helium gas filled in the balloon is
A)3.53 * 1023.
B)5.42 * 1023.
C)9.2 * 1022.
D)7.1 * 1022.
E)4.9 * 1021.
A)3.53 * 1023.
B)5.42 * 1023.
C)9.2 * 1022.
D)7.1 * 1022.
E)4.9 * 1021.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
52
Nuclear fusion is achieved by fusing hydrogen isotopes at high temperatures.The average kinetic energy of deuterium,one of the isotopes of hydrogen,at this temperature is 2.1 *10-14 J.What is the temperature?
A)8.5 * 108 K
B)1.5 * 109 K
C)2.6 * 109 K
D)1.0 * 109 K
E)4.1 * 109 K
A)8.5 * 108 K
B)1.5 * 109 K
C)2.6 * 109 K
D)1.0 * 109 K
E)4.1 * 109 K

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
53
A gas is in a closed container of volume 35 cm3.If its temperature is initially 278 K,by how much do you need to change the temperature in order to increase its pressure from the initial 1 atm to 1.2 atm?
A)+56 K
B)+46 K
C)-46 K
D)-56 K
A)+56 K
B)+46 K
C)-46 K
D)-56 K

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
54
A cylinder with a piston contains 2.00 liters of an ideal diatomic gas at 1.00 atm and 275 K.The gas is subject to an adiabatic compression from its initial volume to 1/4 its initial volume.What is the temperature after the compression?
A)479 K
B)693 K
C)1920 K
D)2770 K
A)479 K
B)693 K
C)1920 K
D)2770 K

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
55
Nuclear fusion is achieved by fusing hydrogen isotopes at high temperatures of the order of 109 K.The average kinetic energy of deuterium,one of the isotopes of hydrogen,at this temperature is
A)2.07 * 10-14 J.
B)4.1 * 10-14 J.
C)6.7 * 10-13 J.
D)9.8 * 10-12 J.
E)11.5 * 10-12 J.
A)2.07 * 10-14 J.
B)4.1 * 10-14 J.
C)6.7 * 10-13 J.
D)9.8 * 10-12 J.
E)11.5 * 10-12 J.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
56
Two identical containers hold equal masses of oxygen in one and nitrogen in the other.The gasses are held at the same temperature.How does the pressure of oxygen compare to that of nitrogen?
A)PO > PN
B)PO = PN
C)PO < PN
D)It cannot be determined.
A)PO > PN
B)PO = PN
C)PO < PN
D)It cannot be determined.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
57
A cylinder with a piston contains 2.00 liters of an ideal monatomic gas at 1.00 atm and 275 K.The gas is subject to an adiabatic compression from its initial volume to 1/4 its initial volume.What is the pressure after the compression?
A)6.96 atm
B)10.1 atm
C)2.52 atm
D)1.74 atm
A)6.96 atm
B)10.1 atm
C)2.52 atm
D)1.74 atm

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
58
The diameter of an oxygen molecule is about 0.3 nm.If the pressure is atmospheric pressure and the mean free path is 1.0 m,what is the temperature?
A)3200 K
B)3400 K
C)3600 K
D)2900 K
E)273 K
A)3200 K
B)3400 K
C)3600 K
D)2900 K
E)273 K

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
59
Two containers contain the same gas at different temperatures and pressures as detailed in the drawing.The small container has a volume of 1 liter and the large container has a volume of 2 liters.The two containers are then connected to each other using a thin tube and the system is allowed to reach equal pressure and temperature in both containers.If the final temperature is 300 K,what is the final pressure? You may assume that the connecting tube has negligible volume and mass. 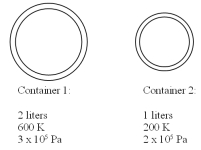
A)400 kPa
B)250 kPa
C)200 kPa
D)300 kPa

A)400 kPa
B)250 kPa
C)200 kPa
D)300 kPa

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
60
The diameter of an oxygen molecule is about 0.3 nm.What is the mean free path at 300 K and 100 kPa?
A)120 nm
B)20 nm
C)80 nm
D)100 nm
E)50 nm
A)120 nm
B)20 nm
C)80 nm
D)100 nm
E)50 nm

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
61
The pressure of an ideal gas is tripled and its volume is decreased to 25% of its original value.How is the temperature affected?
A)T' = 3/4 T
B)T' = 4/3 T
C)T' = (3/4)1/2 T
D)T' = (4/3)1/2 T
E)T' = (3/4)2 T
A)T' = 3/4 T
B)T' = 4/3 T
C)T' = (3/4)1/2 T
D)T' = (4/3)1/2 T
E)T' = (3/4)2 T

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
62
One hundred grams of liquid argon is sealed inside of a 2.0 liter container.After the liquid argon turns into a gas and the gas reaches a temperature of 0.0 degrees Celsius,what is the pressure inside the container due to the liquid argon?
A)0.07 atm
B)0.10 atm
C)4.5 atm
D)7.0 atm
E)28 atm
A)0.07 atm
B)0.10 atm
C)4.5 atm
D)7.0 atm
E)28 atm

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
63
There are two vessels;one contains twice as many molecules as the other but has the same volume.
A)They must have the same pressure.
B)They must have the same temperature.
C)At the same pressure,the one with more molecules has 2 times the temperature (K).
D)If they have the same pressure,the one with more molecules has 1/2 the temperature (K).
E)They must both have the same temperature and pressure.
A)They must have the same pressure.
B)They must have the same temperature.
C)At the same pressure,the one with more molecules has 2 times the temperature (K).
D)If they have the same pressure,the one with more molecules has 1/2 the temperature (K).
E)They must both have the same temperature and pressure.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
64
A scuba diver at a depth of 20 m exhales,releasing a spherical bubble of gas.The bubble floats towards the surface maintaining its spherical shape.When the bubble is 1.0 m below the surface,its diameter is 17 cm.What was the diameter of the air bubble just as it left the diver?
A)10 cm
B)17 cm
C)14 cm
D)12 cm
E)15 cm
A)10 cm
B)17 cm
C)14 cm
D)12 cm
E)15 cm

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
65
A piston with a volume of 24 liters contains 3.0 moles of argon.It is heated from 20 degrees Celsius to 178 degrees Celsius,and the piston is allowed to expand during the heating process so that the pressure on the gas remains constant.How much heat must be added to the gas to heat it?
A)1200 J
B)5900 J
C)9900 J
D)11000 J
E)18000 J
A)1200 J
B)5900 J
C)9900 J
D)11000 J
E)18000 J

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
66
Air in an engine cylinder is quickly compressed from an initial temperature of 10 C,an initial pressure of 1.0 atmosphere,and an initial volume of 250 cm3 to a final volume of 30 cm3.Assuming the air to be an ideal diatomic gas,find the final temperature of the air.
A)23 degrees Celsius
B)390 degrees Celsius
C)660 degrees Celsius
D)2100 degrees Celsius
E)5200 degrees Celsius
A)23 degrees Celsius
B)390 degrees Celsius
C)660 degrees Celsius
D)2100 degrees Celsius
E)5200 degrees Celsius

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
67
A scuba diver exhales,releasing a spherical bubble of gas with a diameter of 10 cm.The bubble floats towards the surface maintaining its spherical shape.When the bubble is 1.0 m below the surface,its diameter is 16 cm.How far below the water's surface is the diver?
A)15 m
B)36 m
C)28 m
D)45 m
E)20 m
A)15 m
B)36 m
C)28 m
D)45 m
E)20 m

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
68
Two moles of helium are initially at 23 C and 1 atm.The work done by the gas at constant pressure when the volume is doubled is
A)7.5 kJ.
B)4.92 kJ.
C)2.19 kJ.
D)1.56 kJ.
E)0.98 kJ.
A)7.5 kJ.
B)4.92 kJ.
C)2.19 kJ.
D)1.56 kJ.
E)0.98 kJ.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
69
A sealed glass bottle containing air at atmospheric pressure is tossed into an open fire.Assuming that no air escapes,and that the bottle's deformation is negligible,which of the following diagrams best describes the changes of the conditions of the air inside the bottle? 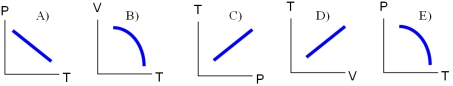
A)diagram A)
B)diagram B)
C)diagram C)
D)diagram D)
E)diagram E)

A)diagram A)
B)diagram B)
C)diagram C)
D)diagram D)
E)diagram E)

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
70
The temperature of an ideal gas is doubled (in K)and its volume is decreased to 25% of its original value.How is the pressure affected?
A)P'=P/2
B)P'=2P
C)P'=4P
D)P'=8P
E)P'=P/4
A)P'=P/2
B)P'=2P
C)P'=4P
D)P'=8P
E)P'=P/4

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
71
Two containers of equal volume contain different ideal gases at the same temperature and pressure.It follows that
A)both containers hold the same number of molecules.
B)the total mass in the two containers is equal.
C)the average speed of the molecules of the two gases is equal.
D)the density of the two gases is equal.
E)the average momenta of the molecules of the two gases is equal.
A)both containers hold the same number of molecules.
B)the total mass in the two containers is equal.
C)the average speed of the molecules of the two gases is equal.
D)the density of the two gases is equal.
E)the average momenta of the molecules of the two gases is equal.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
72
If a gas is in equilibrium at 19 C and is then heated to 38 C,what is the ratio of the average molecular speed to its previous value?
A)38/19
B)311/292
C)(311/292)1/2
D)(311/292)2
E)(38/19)2
A)38/19
B)311/292
C)(311/292)1/2
D)(311/292)2
E)(38/19)2

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
73
What is the rms speed for a collection of helium-3 atoms at 47 degrees Celsius in a container with a volume of 5.0 liters?
A)620 m/s
B)730 m/s
C)1400 m/s
D)1600 m/s
E)2000 m/s
A)620 m/s
B)730 m/s
C)1400 m/s
D)1600 m/s
E)2000 m/s

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
74
A diesel engine takes in air ( = 1.4)at 20 C and compresses it adiabatically to 1/10 of its original volume.The final temperature of the air is
A)592 C.
B)509 C.
C)463 C.
D)375 C.
E)273 C.
A)592 C.
B)509 C.
C)463 C.
D)375 C.
E)273 C.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
75
1.5 liters of nitrogen at 112 C and 2.8 atmospheres contain how many moles?
A)0.13
B)2.8
C)4.5
D)22.4
E)314
A)0.13
B)2.8
C)4.5
D)22.4
E)314

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
76
If,while the temperature is kept constant,the pressure of a diatomic gas doubles,the average kinetic energy of the molecules
A)doubles.
B)increases by less than a factor of two.
C)increases by more than a factor of two.
D)decreases by a factor of two.
E)remains the same.
A)doubles.
B)increases by less than a factor of two.
C)increases by more than a factor of two.
D)decreases by a factor of two.
E)remains the same.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
77
A cylindrical drinking glass with diameter 8.6 cm and height 25 cm is inverted and carefully lowered into a tank of water.If no air is allowed to escape from the glass as it is submerged,what is the height of the air column in the glass when the glass is 2.0 m below the surface of the water?
A)20.9 cm
B)12.5 cm
C)4.10 cm
D)25.0 cm
E)15.2 cm
A)20.9 cm
B)12.5 cm
C)4.10 cm
D)25.0 cm
E)15.2 cm

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
78
A scuba diver at a depth of 20 m exhales,releasing a spherical bubble of gas with a diameter of 10 cm.The bubble floats towards the surface and assuming that it maintains its spherical shape,what is its diameter when it is 1.0 m below the surface?
A)7.8 cm
B)27 cm
C)10 cm
D)14 cm
E)54 cm
A)7.8 cm
B)27 cm
C)10 cm
D)14 cm
E)54 cm

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following statements is true about the average speed of an oxygen molecule,O2,and that of a N2 molecule in a gas at fixed temperature and pressure? MO =16,MN =14
A)The average speed of the O2 molecule is higher.
B)At the same temperature,the average speed of the two molecules is the same.
C)The average speed of the N2 molecule must be lower.
D)The ratio of the average speed of the O2 molecule to that of the N2 molecule must be (32/28)1/2.
E)The ratio of the average speed of the O2 molecule to that of the N2 molecule must be (28/32)1/2.
A)The average speed of the O2 molecule is higher.
B)At the same temperature,the average speed of the two molecules is the same.
C)The average speed of the N2 molecule must be lower.
D)The ratio of the average speed of the O2 molecule to that of the N2 molecule must be (32/28)1/2.
E)The ratio of the average speed of the O2 molecule to that of the N2 molecule must be (28/32)1/2.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
80
The cylinder shown has a radius of 6.0 cm.The current pressure of the gas inside is 2.0 atm.The piston is then pushed in by 20 cm,which is 1/3 of the length of the cylinder.If the temperature remains constant,what is the new pressure of the gas? 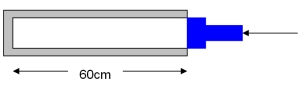
A)0.33 atm
B)1.33 atm
C)3.00 atm
D)3.33 atm
E)6.67 atm

A)0.33 atm
B)1.33 atm
C)3.00 atm
D)3.33 atm
E)6.67 atm

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck


