Deck 4: Three-Dimensional Geometry,intermolecular Interactions,and Physical Properties
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/53
Play
Full screen (f)
Deck 4: Three-Dimensional Geometry,intermolecular Interactions,and Physical Properties
1
Which of the following molecules contains a trigonal planar nitrogen atom connected to two different tetrahedral carbon atoms? 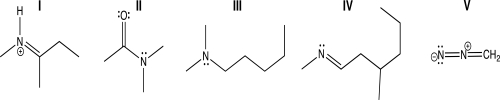
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V
II
2
Which of the following molecules contains a nitrogen atom that has bent molecular geometry? 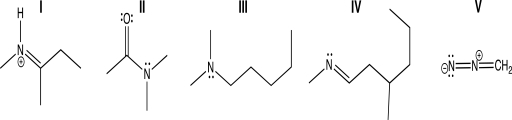
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V
IV
3
The carbon atoms in the molecule below are labeled 1-8.Which CCC bond angle in the molecule would be approximately 120°? 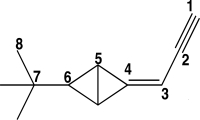
A)C1-C2-C3
B)C2-C3-C4
C)C4-C5-C6
D)C5-C6-C7
E)C6-C7-C8

A)C1-C2-C3
B)C2-C3-C4
C)C4-C5-C6
D)C5-C6-C7
E)C6-C7-C8
C2-C3-C4
4
Rotate the molecule below 180°,in the same way you would flip a pancake or an egg during cooking.Which choice represents the product of the manipulation? 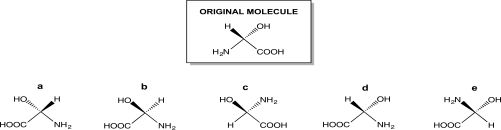
A)Structure a
B)Structure b
C)Structure c
D)Structure d
E)Structure e

A)Structure a
B)Structure b
C)Structure c
D)Structure d
E)Structure e

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
5
Turn the original molecule shown below 90° in a clockwise direction on the plane of this paper.Which choice represents the product of this manipulation? 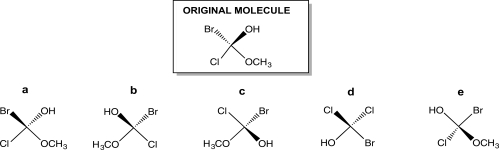
A)Structure a
B)Structure b
C)Structure c
D)Structure d
E)Structure e

A)Structure a
B)Structure b
C)Structure c
D)Structure d
E)Structure e

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
6
What is the VSEPR geometry for the carbon atom of a carbonyl?
A)Linear
B)Tetrahedral
C)Trigonal pyramidal
D)Trigonal planar
E)Bent
A)Linear
B)Tetrahedral
C)Trigonal pyramidal
D)Trigonal planar
E)Bent

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following cycloalkanes contains a CCC bond angle of approximately 90°?
A)A seven-membered cycloalkane
B)A six-membered cycloalkane
C)A five-membered cycloalkane
D)A four-membered cycloalkane
A)A seven-membered cycloalkane
B)A six-membered cycloalkane
C)A five-membered cycloalkane
D)A four-membered cycloalkane

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following molecules contain(s)a nitrogen atom that has trigonal pyramidal molecular geometry? 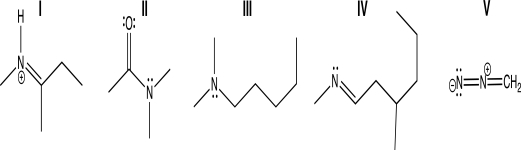
A)I only
B)II only
C)III only
D)I and II
E)II and III

A)I only
B)II only
C)III only
D)I and II
E)II and III

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
9
When applying VSEPR theory to determine the geometry about a central atom,it is important to count the number of electron groups.Separately consider the two atoms highlighted with an arrow in the molecule shown below.How many electron groups must be considered for each of these central atoms? 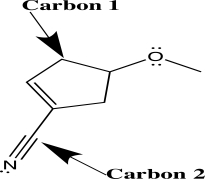
A)C1 has two groups; C2 has two groups.
B)C1 has three groups; C2 has four groups.
C)C1 has four groups; C2 has two groups.
D)C1 has four groups; C2 has three groups.
E)C1 has four groups; C2 has four groups.

A)C1 has two groups; C2 has two groups.
B)C1 has three groups; C2 has four groups.
C)C1 has four groups; C2 has two groups.
D)C1 has four groups; C2 has three groups.
E)C1 has four groups; C2 has four groups.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
10
The carbon atoms in the molecule below are labeled 1-8.Which CCC bond angle in the molecule would be approximately 180°? 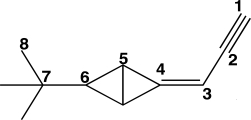
A)C1-C2-C3
B)C2-C3-C4
C)C4-C5-C6
D)C5-C6-C7
E)C6-C7-C8

A)C1-C2-C3
B)C2-C3-C4
C)C4-C5-C6
D)C5-C6-C7
E)C6-C7-C8

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
11
Which cycloalkane contains a CCC bond angle that deviates from the ideal tetrahedral bond angle by approximately 20°?
A)A seven-membered cycloalkane
B)A six-membered cycloalkane
C)A five-membered cycloalkane
D)A four-membered cycloalkane
E)A three-membered cycloalkane
A)A seven-membered cycloalkane
B)A six-membered cycloalkane
C)A five-membered cycloalkane
D)A four-membered cycloalkane
E)A three-membered cycloalkane

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following statements is true about carbon tetrachloride,CCl4?
A)It is polar protic with tetrahedral geometry.
B)The carbon has trigonal planar geometry.
C)None of the CCl bonds has a dipole.
D)It is miscible in water with 109.5° bond angles.
E)It is polar aprotic with tetrahedral geometry at C.
A)It is polar protic with tetrahedral geometry.
B)The carbon has trigonal planar geometry.
C)None of the CCl bonds has a dipole.
D)It is miscible in water with 109.5° bond angles.
E)It is polar aprotic with tetrahedral geometry at C.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following molecules contains a nitrogen atom with linear geometry? 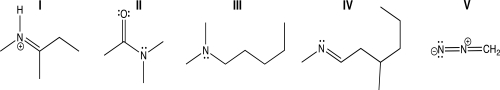
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
14
What are the approximate HCH bond angles expected for the carbanion whose structure is given in the ball-and-stick representation below? 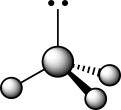
A)180°
B)150°
C)109.5°
D)107°
E)90°

A)180°
B)150°
C)109.5°
D)107°
E)90°

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following molecules possesses at least one polar covalent bond but does not have an overall net molecular dipole?
A)CH4
B)CHCl3
C)CH2Cl2
D)CCl4
E)CH3CH3
A)CH4
B)CHCl3
C)CH2Cl2
D)CCl4
E)CH3CH3

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
16
The carbon atoms in the molecule below are labeled 1-8.Which CCC bond angle in the molecule would be approximately 109.5°? 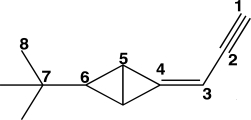
A)C1-C2-C3
B)C2-C3-C4
C)C4-C5-C6
D)C5-C6-C7
E)C6-C7-C8

A)C1-C2-C3
B)C2-C3-C4
C)C4-C5-C6
D)C5-C6-C7
E)C6-C7-C8

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
17
Which cycloalkane has the greatest ring strain per-CH2-unit?
A)A seven-membered cycloalkane
B)A six-membered cycloalkane
C)A five-membered cycloalkane
D)A four-membered cycloalkane
E)A three-membered cycloalkane
A)A seven-membered cycloalkane
B)A six-membered cycloalkane
C)A five-membered cycloalkane
D)A four-membered cycloalkane
E)A three-membered cycloalkane

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
18
Which cycloalkane contains a CCC bond angle that deviates from the ideal tetrahedral bond angle by approximately 50°?
A)A seven-membered cycloalkane
B)A six-membered cycloalkane
C)A five-membered cycloalkane
D)A four-membered cycloalkane
E)A three-membered cycloalkane
A)A seven-membered cycloalkane
B)A six-membered cycloalkane
C)A five-membered cycloalkane
D)A four-membered cycloalkane
E)A three-membered cycloalkane

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following choices correctly describes the structure of the ball-and-stick representation with the formula H3C+? 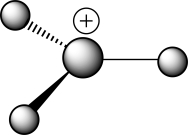
A)A carbocation with a tetrahedral carbon
B)A carbocation with trigonal planar geometry
C)A carbocation with unknown geometry
D)A carbanion with a tetrahedral carbon
E)A carbanion with trigonal planar geometry

A)A carbocation with a tetrahedral carbon
B)A carbocation with trigonal planar geometry
C)A carbocation with unknown geometry
D)A carbanion with a tetrahedral carbon
E)A carbanion with trigonal planar geometry

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
20
What is the VSEPR geometry for any carbon atom in a phenyl ring?
A)Linear
B)Tetrahedral
C)Trigonal pyramidal
D)Trigonal planar
E)Bent
A)Linear
B)Tetrahedral
C)Trigonal pyramidal
D)Trigonal planar
E)Bent

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
21
Are any of the four 1,2-difluorocyclopropane isomers drawn below polar? Indicate the direction of the net molecular dipole,if one is present. 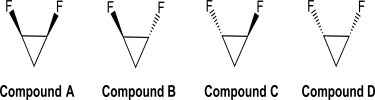


Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
22
How does the presence of the lone pair affect the geometry of the central atom in the following molecule? 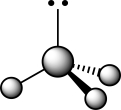
I The lone pair is attracted to the nuclei of the three substituents,creating larger bond angles.
II The lone pair repels the three sets of covalently bonded electrons.
III The lone pair has no bearing whatsoever on the VSEPR geometry at the central atom.
IVThe bond angles are smaller than a traditional tetrahedral bond angle due to lone pair repulsion.
A)I
B)II
C)III
D)IV
E)II and IV

I The lone pair is attracted to the nuclei of the three substituents,creating larger bond angles.
II The lone pair repels the three sets of covalently bonded electrons.
III The lone pair has no bearing whatsoever on the VSEPR geometry at the central atom.
IVThe bond angles are smaller than a traditional tetrahedral bond angle due to lone pair repulsion.
A)I
B)II
C)III
D)IV
E)II and IV

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
23
Sodium chloride,an ionic compound,is highly water soluble but minimally soluble in the polar aprotic solvent dimethyl sulfoxide (DMSO).Why?

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
24
What is the strongest intermolecular attractive force possible between an alkyl chloride and an alkane?
A)Hydrogen bond
B)Ion-dipole
C)Ion-ion
D)Dipole-induced dipole
E)Induced dipole-induced dipole
A)Hydrogen bond
B)Ion-dipole
C)Ion-ion
D)Dipole-induced dipole
E)Induced dipole-induced dipole

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
25
Identify the weakest intermolecular force.
A)Hydrogen bond
B)Ion-dipole
C)Ion-ion
D)Dipole-induced dipole
E)Induced dipole-induced dipole
A)Hydrogen bond
B)Ion-dipole
C)Ion-ion
D)Dipole-induced dipole
E)Induced dipole-induced dipole

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
26
Rank the following molecules based on decreasing boiling point. 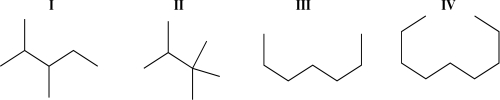
A)I > II > III > IV
B)II > I > III > IV
C)I > III > IV > II
D)IV > III > I > II
E)IV > III > II > I

A)I > II > III > IV
B)II > I > III > IV
C)I > III > IV > II
D)IV > III > I > II
E)IV > III > II > I

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
27
A reverse micelle can form when a polar substance dissolves in a nonpolar substance.Create a conceptual diagram of a reverse micelle.What intermolecular forces are driving the formation of the reverse micelle?

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
28
How many hydrogen-bond donors and acceptors are present in the following molecule? 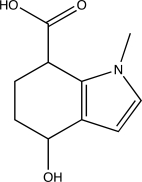
A)One donor and four acceptors
B)Two donors and four acceptors
C)Two donors and three acceptors
D)One donor and three acceptors
E)Two donors and two acceptors

A)One donor and four acceptors
B)Two donors and four acceptors
C)Two donors and three acceptors
D)One donor and three acceptors
E)Two donors and two acceptors

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
29
Dimethyl sulfoxide (DMSO)is a polar aprotic solvent that is frequently used for organic reactions.Rank the following sodium halide salts for decreasing solubility in DMSO.
A)NaBr > NaCl > NaI
B)NaBr > NaI > NaCl
C)NaCl > NaI > NaBr
D)NaCl > NaBr > NaI
E)None of these salts is soluble in DMSO.
A)NaBr > NaCl > NaI
B)NaBr > NaI > NaCl
C)NaCl > NaI > NaBr
D)NaCl > NaBr > NaI
E)None of these salts is soluble in DMSO.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
30
When mixed,which of the following pairs of compounds will exhibit both ion-dipole and ion-ion intermolecular attractive forces? 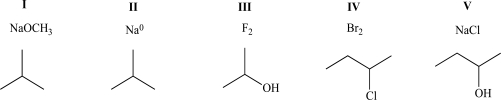
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
31
Which functional group will engage in dipole-dipole interactions,but will not serve as a hydrogen-bond acceptor?
A)Nitrile
B)Ketone
C)Alkyl bromide
D)Carboxylic acid
E)Amine
A)Nitrile
B)Ketone
C)Alkyl bromide
D)Carboxylic acid
E)Amine

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
32
Consider the structure of sodium benzoate,NaOC(O)Ph,the sodium salt of benzoic acid.In which of the following solvents would you predict sodium benzoate to be soluble?
IWater,H2O
IIPentane,CH3(CH2)3CH3
IIIDiethyl ether,(CH3CH2)2O
IVMethanol,CH3OH
VAcetone,CH3C(O)CH3
A)I only
B)I and III
C)I and IV
D)III and V
E)III and IV
IWater,H2O
IIPentane,CH3(CH2)3CH3
IIIDiethyl ether,(CH3CH2)2O
IVMethanol,CH3OH
VAcetone,CH3C(O)CH3
A)I only
B)I and III
C)I and IV
D)III and V
E)III and IV

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following functional groups contains both a hydrogen-bond donor and a hydrogen-bond acceptor?
A)Alkyl fluoride
B)Epoxide
C)Carboxylic acid
D)Nitrile
E)Ketone
A)Alkyl fluoride
B)Epoxide
C)Carboxylic acid
D)Nitrile
E)Ketone

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
34
Select a phrase to complete this sentence: "___________________ are induced dipole-induced dipole interactions common to nonpolar molecules such as hydrocarbons."
A)Electromotive forces
B)Hydrogen bonds
C)Ionic attractions
D)London dispersion forces
E)Mechanical forces
A)Electromotive forces
B)Hydrogen bonds
C)Ionic attractions
D)London dispersion forces
E)Mechanical forces

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
35
Add substituents using dash-wedge notation to achieve the structure specified.
(a) An alkene that has a fluorine atom pointing back on the leftmost carbon and a methyl group coming out on the rightmost carbon.Assume that hydrogen fills the valence of carbon.
(b) A tetrahedral carbon with two chlorine atoms pointing down on the plane of the paper,a bromine atom pointing up,and a hydrogen atom pointing back.
(a) An alkene that has a fluorine atom pointing back on the leftmost carbon and a methyl group coming out on the rightmost carbon.Assume that hydrogen fills the valence of carbon.
(b) A tetrahedral carbon with two chlorine atoms pointing down on the plane of the paper,a bromine atom pointing up,and a hydrogen atom pointing back.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
36
What is the strongest intermolecular attractive force between an alcohol and a ketone?
A)Hydrogen bond
B)Ion-dipole
C)Ion-ion
D)Dipole-induced dipole
E)Induced dipole-induced dipole
A)Hydrogen bond
B)Ion-dipole
C)Ion-ion
D)Dipole-induced dipole
E)Induced dipole-induced dipole

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
37
When applying VSEPR theory to determine the geometry about a central atom,it is important to count the total number of bonded and nonbonded electron groups.Separately consider the two atoms highlighted with an arrow in the molecule shown below.How many bonded electron groups must be considered for each of these central atoms? 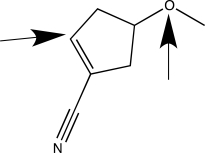
A)C has two groups; O has two groups.
B)C has three groups; O has four groups.
C)C has three groups; O has two groups.
D)C has three groups; O has three groups.
E)C has four groups; O has four groups.

A)C has two groups; O has two groups.
B)C has three groups; O has four groups.
C)C has three groups; O has two groups.
D)C has three groups; O has three groups.
E)C has four groups; O has four groups.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
38
Rank the following molecules based on increasing boiling point. 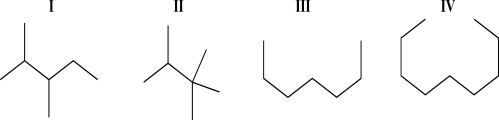
A)I < II < III < IV
B)II < I < III < IV
C)IV < III < I < II
D)II < III < IV < I
E)IV < III < II < I

A)I < II < III < IV
B)II < I < III < IV
C)IV < III < I < II
D)II < III < IV < I
E)IV < III < II < I

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following intermolecular forces is responsible for the boiling-point trends in alkanes?
A)Hydrogen bond
B)Ion-dipole
C)Ion-ion
D)Dipole-induced dipole
E)Induced dipole-induced dipole
A)Hydrogen bond
B)Ion-dipole
C)Ion-ion
D)Dipole-induced dipole
E)Induced dipole-induced dipole

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
40
Identify the strongest intermolecular force.
A)Hydrogen bond
B)Ion-dipole
C)Ion-ion
D)Dipole-induced dipole
E)Induced dipole-induced dipole
A)Hydrogen bond
B)Ion-dipole
C)Ion-ion
D)Dipole-induced dipole
E)Induced dipole-induced dipole

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
41
Rank N-N-dimethylaniline,phenethylamine,and phenethylamine hydrochloride in order of decreasing boiling point.Explain your reasoning.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
42
Explain the chemical difference between a detergent and a soap.Provide one example of each in your answer.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following benzene derivatives would be most soluble in benzene? Why? 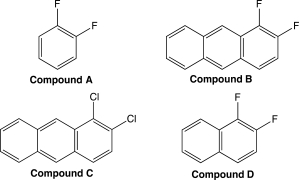


Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
44
Propanol can be dissolved in diethyl ether and treated with sodium hydride,a base,to form a sodium alkoxide,as shown below. 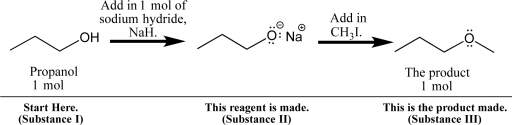
Although this and related chemical reactions will be studied in later chapters,you can apply your knowledge of solubility to the process.
Fill in your solubility predictions using the table below.Do you expect propanol (I),sodium propoxide (II),and the new ether product (III)to be soluble in the reaction solvent,diethyl ether? If soluble,predict the intermolecular interactions that will exist in solution.Also,anticipate the physical appearance of the reaction at each point: Will the solution be transparent,or will a precipitate form?


Although this and related chemical reactions will be studied in later chapters,you can apply your knowledge of solubility to the process.
Fill in your solubility predictions using the table below.Do you expect propanol (I),sodium propoxide (II),and the new ether product (III)to be soluble in the reaction solvent,diethyl ether? If soluble,predict the intermolecular interactions that will exist in solution.Also,anticipate the physical appearance of the reaction at each point: Will the solution be transparent,or will a precipitate form?


Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
45
Your lab partner disobeyed lab rules and poured table salt from lunch into your unknown organic white powder,which is aprotic and contains an ester functional group.What solvent might you add to the solid mixture to remove the table salt,leaving the ester? What solvent is appropriate to remove the unknown ester,leaving instead the salt? Explain your reasoning.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
46
(a) Group I cations are common ions found in organic salts.Write the electron configuration for the Group I cations below.In the fourth column,use circles to represent the relative size of each cation conceptually.Define the term ionic radius and label the ionic radius of each cation.
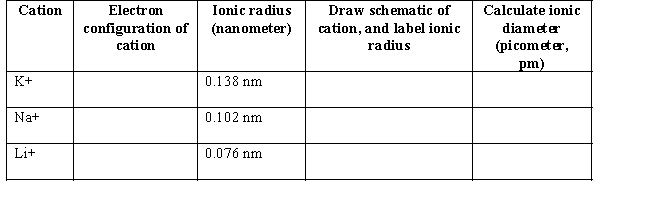
(b) Organic molecules called crown ethers (refer to the box titled "Phase Transfer Catalysts" in Chapter 2 of your text)can sequester a cation of specific size to make the organic anion more reactive.Charles Pedersen,in fact,shared the 1987 Nobel Prize in Chemistry for contributions to the synthesis of crown ethers.Suppose you wanted to use a crown ether to selectively remove each individual cation from a solution of sodium,lithium,and potassium.For each cation,which crown ether might you add?
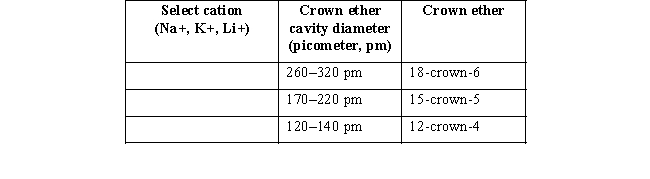

(b) Organic molecules called crown ethers (refer to the box titled "Phase Transfer Catalysts" in Chapter 2 of your text)can sequester a cation of specific size to make the organic anion more reactive.Charles Pedersen,in fact,shared the 1987 Nobel Prize in Chemistry for contributions to the synthesis of crown ethers.Suppose you wanted to use a crown ether to selectively remove each individual cation from a solution of sodium,lithium,and potassium.For each cation,which crown ether might you add?


Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
47
Rank 1,4-dimethylbenzene,phenol,and N,N-dimethylaniline in order of decreasing solubility in the organic solvent toluene.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
48
Why do polar aprotic solvents solvate cations more strongly than anions? Using sodium chloride as the representative ionic compound,diagram the intermolecular interactions with tetrahydrofuran (THF).

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
49
Explain why polar protic solvents (like butanol)solvate anions (like chloride)better than polar aprotic solvents (like N,N-dimethylformamide,DMF)do.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
50
The solvent tert-butyl methyl ether (MTBE)is used as a "greener" replacement for the organic solvent diethyl ether,because it has less propensity to form peroxides upon standing.Draw the line structures of both diethyl ether and MTBE.Look up the physical properties (boiling point,flash point)of both solvents.Explain why the boiling point of diethyl ether is lower.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
51
Tertiary amides are typically insoluble in water.The solvent dimethyl acetamide,CH3CON(CH3)2,which is commonly called DMA,is an exception.Explain why the solvent DMA is soluble in water.Diagram the intermolecular forces that make DMA soluble in water.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
52
A micelle is formed when soap dissolves in water.What intermolecular forces govern the formation of the micelle? Explain.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
53
Winter gasoline mixes for cold climates require 10% ethanol,or 1 gallon of ethanol for every
9 gallons of gasoline,in part to help prevent water in the gas from freezing and causing engine trouble.Diagram the intermolecular forces between water and ethanol,and between ethanol and octane.Why are the intermolecular forces relevant in winter mix gasoline?
9 gallons of gasoline,in part to help prevent water in the gas from freezing and causing engine trouble.Diagram the intermolecular forces between water and ethanol,and between ethanol and octane.Why are the intermolecular forces relevant in winter mix gasoline?

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck


