Deck 5: Orbital Interactions 1: Hybridization and Two-Center Molecular Orbitals
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/56
Play
Full screen (f)
Deck 5: Orbital Interactions 1: Hybridization and Two-Center Molecular Orbitals
1
What abbreviation is used to designate the molecular orbital of highest energy that is occupied with electrons?
A)MOHE
B)HOME
C)HOMO
D)HAHA
E)LUMO
A)MOHE
B)HOME
C)HOMO
D)HAHA
E)LUMO
HOMO
2
Which two atomic orbitals overlap to form the MOs of σ symmetry that correspond to the CC bond in ethylene?
A)2s and 2p
B)2p and sp2
C)sp2 and sp2
D)sp3 and sp3
E)2p and 2p
A)2s and 2p
B)2p and sp2
C)sp2 and sp2
D)sp3 and sp3
E)2p and 2p
sp2 and sp2
3
Which CC bond in the molecule below is formed from overlap of an sp3 orbital and an sp2 orbital? 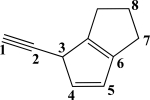
A)The C1C2 σ bond
B)The C1C2 π bond
C)The C3C4 σ bond
D)The C3C4 π bond
E)The C4C5 σ bond

A)The C1C2 σ bond
B)The C1C2 π bond
C)The C3C4 σ bond
D)The C3C4 π bond
E)The C4C5 σ bond
The C3C4 σ bond
4
Which CC bond in the molecule below is formed from overlap of two 2p orbitals? 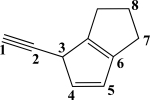
A)The C1C2 σ bond
B)The C1C2 π bond
C)The C3C4 σ bond
D)The C3C4 π bond
E)The C4C5 σ bond

A)The C1C2 σ bond
B)The C1C2 π bond
C)The C3C4 σ bond
D)The C3C4 π bond
E)The C4C5 σ bond

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
5
Which CC bond in the molecule below is formed from overlap of two sp2-hybridized orbitals? 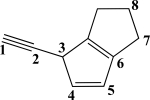
A)The C6C7 σ bond
B)The C7 C8 σ bond
C)The C5C6 σ bond
D)The C4C5 π bond
E)The C4C5 σ bond

A)The C6C7 σ bond
B)The C7 C8 σ bond
C)The C5C6 σ bond
D)The C4C5 π bond
E)The C4C5 σ bond

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
6
Boat A follows Boat B across a lake,keeping a safe distance.The waves generated by the two boats meet to create a new wave that is unusually large.What chemical concept explains the formation of the larger wave?
A)Destructive interference occurs.
B)Constructive interference occurs.
C)Molecular orbital interference occurs.
D)The Heisenberg uncertainty principle dominates.
E)Hund's rule plays a role.
A)Destructive interference occurs.
B)Constructive interference occurs.
C)Molecular orbital interference occurs.
D)The Heisenberg uncertainty principle dominates.
E)Hund's rule plays a role.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
7
Which two carbon atoms participate in the strongest σ bond? 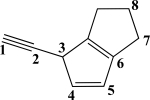
A)C1C2
B)C2C3
C)C3C4
D)C4C5
E)C7C8

A)C1C2
B)C2C3
C)C3C4
D)C4C5
E)C7C8

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following statements about molecular orbital theory are true?
I. According to MO theory,electrons are localized on specific atoms.
II.The linear combination of atomic orbitals method is used to generate molecular orbitals for a molecule.
III. The number of molecular orbitals created from the LCAO approach is identical to the number of atomic orbitals that were originally combined.
IV. Molecular orbital theory is a powerful bonding theory that accurately predicts structures of complex molecules.
V. The MOs generated are classified as either bonding,nonbonding,or antibonding.
A)Only II, III, and IV are true.
B)Only III, IV, and V are true.
C)Only IV and V are true.
D)All choices except I are true.
E)All choices are true.
I. According to MO theory,electrons are localized on specific atoms.
II.The linear combination of atomic orbitals method is used to generate molecular orbitals for a molecule.
III. The number of molecular orbitals created from the LCAO approach is identical to the number of atomic orbitals that were originally combined.
IV. Molecular orbital theory is a powerful bonding theory that accurately predicts structures of complex molecules.
V. The MOs generated are classified as either bonding,nonbonding,or antibonding.
A)Only II, III, and IV are true.
B)Only III, IV, and V are true.
C)Only IV and V are true.
D)All choices except I are true.
E)All choices are true.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
9
Which characteristics describe the bonding MO for the weakest individual covalent bond in ethene,C2H4?
I. The MO possesses π symmetry.
II.The MO possesses σ symmetry.
III.The MO can be called the HOMO.
IV.The MO can be called the LUMO.
V.The MO contains two electrons of opposing spin.
A)All of the choices
B)II, III, and V
C)II and V
D)I and V
E)I, III, and V
I. The MO possesses π symmetry.
II.The MO possesses σ symmetry.
III.The MO can be called the HOMO.
IV.The MO can be called the LUMO.
V.The MO contains two electrons of opposing spin.
A)All of the choices
B)II, III, and V
C)II and V
D)I and V
E)I, III, and V

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
10
According to valence bond theory,which atomic orbitals of carbon may be hybridized to account for bonding? Why?
A)1s, 2s, and 2p orbitals all may hybridize; these orbitals are of the same phase.
B)Only 2p orbitals may hybridize; the 2p orbital is carbon's highest-energy atomic orbital.
C)Only 1s and 2s orbitals of carbon may hybridize; each contains electrons to share in bonding.
D)Only 2s and 2p may hybridize; these orbitals contain valence electrons used in bonding.
E)Only 2s orbitals may hybridize; these orbitals contain valence electrons.
A)1s, 2s, and 2p orbitals all may hybridize; these orbitals are of the same phase.
B)Only 2p orbitals may hybridize; the 2p orbital is carbon's highest-energy atomic orbital.
C)Only 1s and 2s orbitals of carbon may hybridize; each contains electrons to share in bonding.
D)Only 2s and 2p may hybridize; these orbitals contain valence electrons used in bonding.
E)Only 2s orbitals may hybridize; these orbitals contain valence electrons.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
11
Which two carbon atoms participate in the longest σ bond? 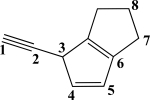
A)C1C2
B)C2C3
C)C3C4
D)C4C5
E)C7C8

A)C1C2
B)C2C3
C)C3C4
D)C4C5
E)C7C8

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
12
Naltrexone is an antagonist at the mu opioid receptor.What is the hybridization state and geometry of the nitrogen atom in naltrexone? 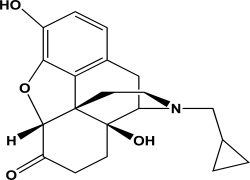
A)sp hybridized and linear geometry
B)sp2 hybridized and trigonal pyramidal
C)sp3 hybridized and trigonal pyramidal
D)sp3 hybridized and trigonal planar
E)sp3 hybridized and bent

A)sp hybridized and linear geometry
B)sp2 hybridized and trigonal pyramidal
C)sp3 hybridized and trigonal pyramidal
D)sp3 hybridized and trigonal planar
E)sp3 hybridized and bent

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following functional groups has a lone pair residing in an sp-hybridized orbital?
A)Amide
B)Alkyne
C)Amine
D)Nitrile
E)Ester
A)Amide
B)Alkyne
C)Amine
D)Nitrile
E)Ester

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
14
An atom's electrons have a high probability of being found within a region of three-dimensional space defined by quantum mechanics.What term identifies this region?
A)Electron configuration
B)Atomic orbital
C)Central core
D)Nucleus
E)Valence shell
A)Electron configuration
B)Atomic orbital
C)Central core
D)Nucleus
E)Valence shell

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following structures contains an sp2-hybridized oxygen atom because a lone pair on oxygen is delocalized via resonance? 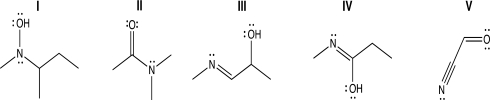
A)Structure I
B)Structure II
C)Structures II and V
D)Structure IV
E)Structures I and IV

A)Structure I
B)Structure II
C)Structures II and V
D)Structure IV
E)Structures I and IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
16
Which functional group has two sp2-hybridized oxygen atoms within its structure,yet cannot be a H-bond donor?
A)Amide
B)Alcohol
C)Ester
D)Ketone
E)Aldehyde
A)Amide
B)Alcohol
C)Ester
D)Ketone
E)Aldehyde

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
17
Boat A and Boat B start on opposite sides of a lake.The boats cross the lake heading directly toward one another at equal rates of speed.Upon meeting,what happens to the waves generated by the two boats?
A)Destructive interference occurs, and thus the waves negate each other, leaving only a ripple.
B)Constructive interference occurs, and thus the new wave formed is larger in size.
C)The Heisenberg uncertainty principle dominates, and thus the outcome of the meeting of the two waves is not concrete.
D)Destructive interference occurs, forming one wave that is much larger in size.
E)Constructive interference occurs, forming one wave that is much larger in size.
A)Destructive interference occurs, and thus the waves negate each other, leaving only a ripple.
B)Constructive interference occurs, and thus the new wave formed is larger in size.
C)The Heisenberg uncertainty principle dominates, and thus the outcome of the meeting of the two waves is not concrete.
D)Destructive interference occurs, forming one wave that is much larger in size.
E)Constructive interference occurs, forming one wave that is much larger in size.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
18
From left to right,identify the hybridization of the three carbon atoms in the interesting organic structure below.These interesting structures are called cumulenes. 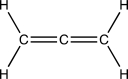
A)sp3, sp2, sp3
B)sp3, sp, sp3
C)sp2, sp, sp2
D)sp, sp, sp2
E)sp2, sp2, sp2
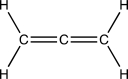
A)sp3, sp2, sp3
B)sp3, sp, sp3
C)sp2, sp, sp2
D)sp, sp, sp2
E)sp2, sp2, sp2

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
19
What abbreviation is used to designate the lowest-energy molecular orbital for a molecule that is devoid of electrons?
A)MOLE
B)LUMO
C)LEMO
D)NEMO
E)MOWO
A)MOLE
B)LUMO
C)LEMO
D)NEMO
E)MOWO

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
20
Rank the highlighted σ bonds in order of decreasing length. 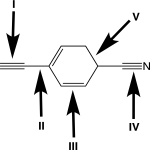
A)I > II > III > IV > V
B)V > III > II > I > IV
C)V > II > III > I > IV
D)V > II > III > IV > I
E)II > V > III > IV > I

A)I > II > III > IV > V
B)V > III > II > I > IV
C)V > II > III > I > IV
D)V > II > III > IV > I
E)II > V > III > IV > I

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
21
How many total electrons reside in molecular orbitals of π symmetry for this molecule? 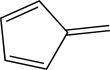
A)Two
B)Three
C)Four
D)Six
E)Eight

A)Two
B)Three
C)Four
D)Six
E)Eight

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
22
Select which of the following molecules have two distinct configurations about the double bond. 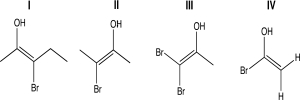
A)All of these
B)All of these except IV
C)All of these except III
D)I and II only
E)II and III only

A)All of these
B)All of these except IV
C)All of these except III
D)I and II only
E)II and III only

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
23
An alkene contains a double bond.Why is the C 
C of an alkene rigid and unable to freely rotate?
A)The C C bond is very strong, and it is impossible to break it.
C bond is very strong, and it is impossible to break it.
B)The σ bond gets blocked by the hydrogen atoms.
C)The sp2 hybrid orbitals of carbon take up too much room.
D)The π electrons are delocalized through the σ bonds.
E)The 2p orbitals of the π bond overlap above and below the C C plane to restrict rotation.

C of an alkene rigid and unable to freely rotate?
A)The C
 C bond is very strong, and it is impossible to break it.
C bond is very strong, and it is impossible to break it.B)The σ bond gets blocked by the hydrogen atoms.
C)The sp2 hybrid orbitals of carbon take up too much room.
D)The π electrons are delocalized through the σ bonds.
E)The 2p orbitals of the π bond overlap above and below the C C plane to restrict rotation.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
24
Given below are 2s,2p,sp,sp2,and sp3 orbitals,all drawn to scale and in no particular order.Which of these orbitals exhibits 50% s character? 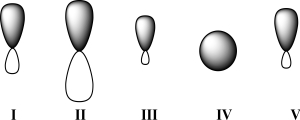
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
25
One of the following statements about the relationship of % s character to organic structure is false.Identify which statement is false.
A)The % s character and effective electronegativity are directly proportional to one another.
B)An sp3 orbital has greater % s character than an sp2 orbital.
C)Two orbitals with high % s character will form a stronger bond.
D)A 2s orbital has greater s character than any of the hybrid orbitals.
E)An orbital with greater % s character is smaller and more compact.
A)The % s character and effective electronegativity are directly proportional to one another.
B)An sp3 orbital has greater % s character than an sp2 orbital.
C)Two orbitals with high % s character will form a stronger bond.
D)A 2s orbital has greater s character than any of the hybrid orbitals.
E)An orbital with greater % s character is smaller and more compact.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
26
Rank the carbon atoms in order of increasing percent s orbital character. 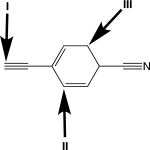
A)I < II < III
B)I < III < II
C)II < III < I
D)II < I < III
E)III < II < I

A)I < II < III
B)I < III < II
C)II < III < I
D)II < I < III
E)III < II < I

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
27
For one or more of the following molecules,(1)two distinct configurations about the double bond are possible,and (2)the -OH and -Br are trans.Select the molecule or molecules that fit this description. 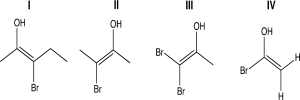
A)I
B)II
C)III
D)IV
E)I and III

A)I
B)II
C)III
D)IV
E)I and III

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
28
Given below are 2s,2p,sp,sp2,and sp3 orbitals,all drawn to scale and in no particular order.Which of these orbitals represents the hybrid orbital with the lowest effective electronegativity? 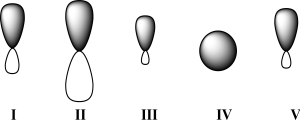
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
29
Given below are 2s,2p,sp,sp2,and sp3 orbitals,all drawn to scale and in no particular order.Which of these orbitals would carbon use in the CO σ bond of acetone? 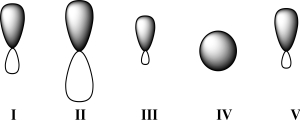
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
30
Given below are 2s,2p,sp,sp2,and sp3 orbitals,all drawn to scale and in no particular order.Which of these orbitals represents the hybrid orbital with the greatest effective electronegativity? 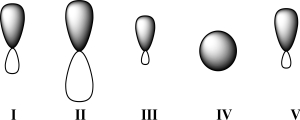
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
31
Styrene is an important industrial chemical in the generation of various plastics.Determine how many bonding molecular orbitals are created during the LCAO process to accommodate the π electrons in styrene. 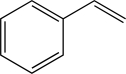
A)Two
B)Four
C)Six
D)Eight
E)Ten

A)Two
B)Four
C)Six
D)Eight
E)Ten

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
32
How many π bonds are present in the molecule below? 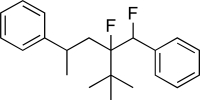
A)Three
B)Four
C)Five
D)Six
E)Seven

A)Three
B)Four
C)Five
D)Six
E)Seven

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
33
Given below are 2s,2p,sp,sp2,and sp3 orbitals,in random order.Which of these orbitals exhibits 33% s character? 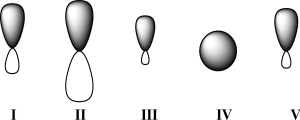
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
34
For one or more of the following molecules,two distinct configurations about the double bond are possible,and the two alkyl substituents are trans.Select the molecule or molecules that fit this description. 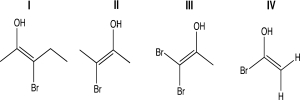
A)I
B)II
C)III
D)IV
E)I and II

A)I
B)II
C)III
D)IV
E)I and II

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
35
Rank the carbon atoms in order of increasing effective electronegativity. 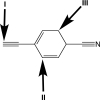
A)I < II < III
B)I < III < II
C)II < III < I
D)II < I < III
E)III < II < I

A)I < II < III
B)I < III < II
C)II < III < I
D)II < I < III
E)III < II < I

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
36
For the given molecule,an amino alcohol,how many electrons are present in nonbonding molecular orbitals? 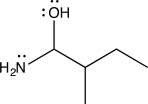
A)Seven
B)Six
C)Five
D)Four
E)Three

A)Seven
B)Six
C)Five
D)Four
E)Three

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
37
Given below are 2s,2p,sp,sp2,and sp3 orbitals,all drawn to scale and in no particular order.Which of these orbitals would carbon use for a CO π bond? 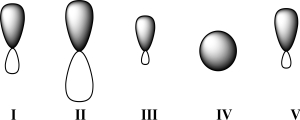
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
38
Which two atomic orbitals overlap to form the MOs of σ symmetry that correspond to a CH bond in ethylene?
A)An sp2 orbital from both C and H
B)A 2p orbital from C and an sp2 orbital from H
C)A 1s orbital of H and the sp2 orbital of C
D)A 2s orbital of H and the sp2 orbital of C
E)A 2p orbital from both C and H
A)An sp2 orbital from both C and H
B)A 2p orbital from C and an sp2 orbital from H
C)A 1s orbital of H and the sp2 orbital of C
D)A 2s orbital of H and the sp2 orbital of C
E)A 2p orbital from both C and H

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
39
What is the geometry and hybridization of the central carbon atom in the cumulene below? 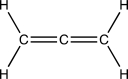
A)sp3 hybridized and tetrahedral
B)sp3 hybridized and trigonal pyramidal
C)sp2 hybridized and trigonal planar
D)sp2 hybridized and bent
E)sp hybridized and linear
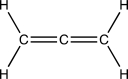
A)sp3 hybridized and tetrahedral
B)sp3 hybridized and trigonal pyramidal
C)sp2 hybridized and trigonal planar
D)sp2 hybridized and bent
E)sp hybridized and linear

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
40
Which two atomic orbitals overlap to form the bonding and antibonding MOs of π symmetry for ethylene,C2H4?
A)2s and 2p
B)2p and sp2
C)sp2 and sp2
D)sp3 and sp3
E)2p and 2p
A)2s and 2p
B)2p and sp2
C)sp2 and sp2
D)sp3 and sp3
E)2p and 2p

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
41
Bioymifi is a novel small molecule that selectively triggers programmed cell death in certain cancer cells.Identify the following structural features in Bioymifi: (a)phenyl ring,(b)a lone pair found in a 2p orbital that can be delocalized three different ways,(c)an sp2-hybridized oxygen whose lone pairs are not delocalized,(d)an sp2-hybridized oxygen whose lone pairs are delocalized,(e)a nitrogen with bent geometry,and (f)a nitrogen with trigonal planar geometry. 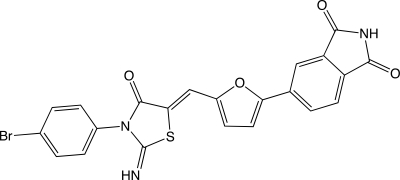


Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
42
Amide bonds join amino acids together to make proteins.Sketch an orbital picture of the amide functional group given below.Identify the orbitals from each atom that are used to form the σ and π bonds in the given amide.Which orbitals house the lone pairs on N and O? 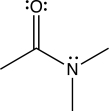


Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
43
Tubulysin D is a peptide-based marine natural product with biological activity.Identify the following structural features of tubulysin D.
(a) Place a box around any sp3 nitrogen.
(b) Star (*)any oxygen atom that has two oxygen lone pairs in sp2-hybridized orbitals.
(c) Place a number symbol (#)on any oxygen atom that is sp2 hybridized as a consequence of having a lone pair in a 2p orbital.
(d) Use an arrow to point to any N atom that has trigonal planar geometry.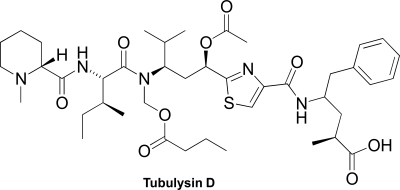
(a) Place a box around any sp3 nitrogen.
(b) Star (*)any oxygen atom that has two oxygen lone pairs in sp2-hybridized orbitals.
(c) Place a number symbol (#)on any oxygen atom that is sp2 hybridized as a consequence of having a lone pair in a 2p orbital.
(d) Use an arrow to point to any N atom that has trigonal planar geometry.


Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
44
Why is a σ bond formed from the overlap of two sp2 orbitals stronger than one formed from the end-on-end overlap of two p orbitals?

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
45
Using line structures,deduce individual resonance contributors from the resonance hybrid structure given here.Identify any lone pairs that are localized,rather than delocalized.Based on orbital hybridization theory,what orbitals accommodate these lone pairs? 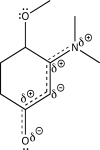


Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
46
Draw line structures of a molecule with the formula C4H6O that has the characteristics outlined in (a),(b),and (c)below.
(a)Two sp-hybridized carbon atoms,a methyl group,and a primary alcohol
(b)An epoxide and one carbon-carbon bond formed from the overlap of 2p orbitals
(c)A cyclopropane ring connected to only one substituent via a σ bond.This σ bond is formed from overlap of one Csp3 and one Csp2 orbital.
(a)Two sp-hybridized carbon atoms,a methyl group,and a primary alcohol
(b)An epoxide and one carbon-carbon bond formed from the overlap of 2p orbitals
(c)A cyclopropane ring connected to only one substituent via a σ bond.This σ bond is formed from overlap of one Csp3 and one Csp2 orbital.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
47
Acetonitrile,C2H3N,is a polar aprotic solvent commonly used in organic reactions.Draw the structure of acetonitrile.Determine the number of σ bonds,the number of π bonds,and the number of electrons occupying nonbonding MOs for the molecule.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
48
Sketch an orbital picture of ethylene,H2C 
CH2,and highlight the orbitals that overlap to form each σ and π bond.Identify the orbitals from each atom that overlap to form the σ and π bonds.Why is the CC π bond weaker than the CC σ bond? Use orbital structure as part of your explanation.

CH2,and highlight the orbitals that overlap to form each σ and π bond.Identify the orbitals from each atom that overlap to form the σ and π bonds.Why is the CC π bond weaker than the CC σ bond? Use orbital structure as part of your explanation.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
49
Formaldehyde,CH2O,is a biological preservative and the simplest aldehyde.Draw the structure of formaldehyde.Determine the number of σ bonds,the number of π bonds,and the number of electrons occupying nonbonding MOs for formaldehyde.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
50
Draw line structures of a molecule with the formula C5H11N that has the characteristics outlined in (a),(b),and (c)below.
(a) An sp2 nitrogen atom with a conjugated lone pair,one methyl substituent,and one ethyl substituent
(b) A disubstituted trans alkene and a primary amine with an sp3 nitrogen connected to an sp3 carbon
(c) A three-membered ring containing a secondary sp3 nitrogen with cis methyl and ethyl substituents on the ring
(a) An sp2 nitrogen atom with a conjugated lone pair,one methyl substituent,and one ethyl substituent
(b) A disubstituted trans alkene and a primary amine with an sp3 nitrogen connected to an sp3 carbon
(c) A three-membered ring containing a secondary sp3 nitrogen with cis methyl and ethyl substituents on the ring

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
51
The molecule benzene,which features a conjugated ring system,has interesting properties.In fact,extended benzene-like molecules are key features of many drug classes,including DNA intercalators.
(a) Draw an energy-based electron configuration diagram for any one carbon atom in benzene.Clearly show which orbitals are hybridized and which orbital is used for the π bond.
(b) Next,consider the π MO diagram of benzene generated via the LCAO method.Add π electrons to the diagram,and label each orbital as π bonding MO or π* antibonding MO.How many total electrons reside in MOs of π symmetry for benzene?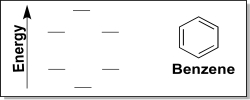
(a) Draw an energy-based electron configuration diagram for any one carbon atom in benzene.Clearly show which orbitals are hybridized and which orbital is used for the π bond.
(b) Next,consider the π MO diagram of benzene generated via the LCAO method.Add π electrons to the diagram,and label each orbital as π bonding MO or π* antibonding MO.How many total electrons reside in MOs of π symmetry for benzene?


Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
52
Use your knowledge of hybridization to fill in the table below.Rank the sigma bonds labeled A,B,C,and D from strongest to weakest.Explain your answer. 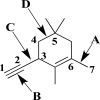



Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
53
Compare the p orbital orientation and overlap needed to form a σ bond and a π bond.Using an example,create a diagram for each type of bond,and differentiate between σ and π bond characteristics.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
54
Consider the hybrid structure below and the labeling scheme provided.Draw the most stable resonance contributor.Identify the hybridization and VSEPR geometry of each atom. 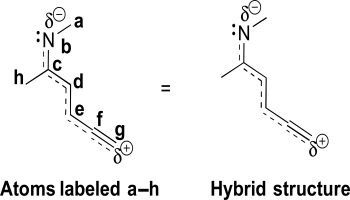


Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
55
Tropyllium bromide is an ionic organic compound that is soluble in water but not in diethyl ether.What is the hybridization of the positively charged carbon in tropyllium bromide? Do you expect the carbon framework to be planar? Justify your response using an orbital diagram. 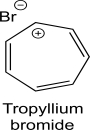


Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
56
Below are two reasonable acyclic structures for ozone,O3,that differ in π electron delocalization.Identify the hybridization of all the oxygen atoms in ozone for each individual resonance contributor.What is the geometry of the central oxygen in each structure?

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck


