Deck 3: Interchapter 1molecular Orbital Theory and Chemical Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/17
Play
Full screen (f)
Deck 3: Interchapter 1molecular Orbital Theory and Chemical Reactions
1
Which of the following transition states will be allowed? 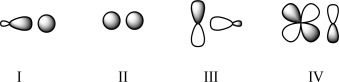
A)II only
B)II and IV
C)III only
D)I, II, and IV
E)I, II, III, and IV

A)II only
B)II and IV
C)III only
D)I, II, and IV
E)I, II, III, and IV
I, II, and IV
2
Draw the energy levels of the transition-state molecular orbitals based on the HOMO-LUMO interaction below. 

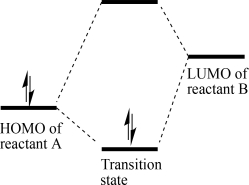
3
Use FMO theory to rationalize the different stereospecificity of SN1 and SN2 reaction mechanisms.
SN2 reactions are stereospecific because the HOMO of the nucleophile (often a nonbonding orbital)must interact with the LUMO of the electrophile (often an antibonding molecular orbital)with the correct symmetry.This can occur only with inversion of stereochemistry.An SN1 reaction proceeds via a planar carbocation intermediate,in which the LUMO is an empty p orbital.The HOMO of the incoming nucleophile can thus interact with either lobe of the p orbital,so a racemic mixture of enantiomers will be obtained.
4
What is meant by the term frontier molecular orbital?

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
5
Draw the HOMO-LUMO interaction that occurs in the transition state of the second step of the reaction shown below (that is,the bromide leaving).Label the HOMO and the LUMO. 


Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
6
Frontier molecular orbital theory can be used to predict which of the following about a chemical reaction?
A)The kinetics of the reaction
B)The thermodynamics of the reaction
C)The stereospecificity of the reaction
D)The stability of the product of the reaction
E)More than one of the above
A)The kinetics of the reaction
B)The thermodynamics of the reaction
C)The stereospecificity of the reaction
D)The stability of the product of the reaction
E)More than one of the above

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
7
According to frontier molecular orbital theory,a reaction is favorable if which of the following is true?
A)The energy gap between the HOMO and the LUMO is large.
B)The HOMO contains nonbonding electrons.
C)The reverse reaction is forbidden.
D)The energy gap between the HOMO and the LUMO is small.
E)The LUMO is at a lower energy than the HOMO.
A)The energy gap between the HOMO and the LUMO is large.
B)The HOMO contains nonbonding electrons.
C)The reverse reaction is forbidden.
D)The energy gap between the HOMO and the LUMO is small.
E)The LUMO is at a lower energy than the HOMO.

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
8
In the elementary reaction step shown below,identify the reactant that contributes the HOMO and the reactant that contributes the LUMO. 


Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
9
According to FMO theory,an allowed transition state requires that the HOMO and the LUMO have which of the following?
A)Similar energies
B)Different energies
C)Similar symmetries only
D)Opposite symmetries
E)Similar energies and symmetries
A)Similar energies
B)Different energies
C)Similar symmetries only
D)Opposite symmetries
E)Similar energies and symmetries

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
10
Draw the HOMO-LUMO interaction that stabilizes the transition state of the 1,2-hydride shift that would occur in the molecule below. 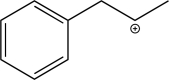


Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
11
Briefly explain why the HOMO and the LUMO must have similar energies in order for a reaction to occur.

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
12
Identify the HOMO and the LUMO that participate in the following reaction. 
A)HOMO = nb orbital on CNLUMO = σ* orbital on CH3Br
B)HOMO = π orbital on CNLUMO = π* orbital on CH3Br
C)HOMO = π orbital on CH3BrLUMO = nb orbital on CN
D)HOMO = nb orbital on CH3BrLUMO = π* orbital on CN
E)HOMO = π orbital on CNLUMO = σ* orbital on CH3Br

A)HOMO = nb orbital on CNLUMO = σ* orbital on CH3Br
B)HOMO = π orbital on CNLUMO = π* orbital on CH3Br
C)HOMO = π orbital on CH3BrLUMO = nb orbital on CN
D)HOMO = nb orbital on CH3BrLUMO = π* orbital on CN
E)HOMO = π orbital on CNLUMO = σ* orbital on CH3Br

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
13
Would the transition state shown below lead to a successful reaction? Why or why not? 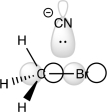


Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
14
Construct the MO energy-level diagram for acetone,and then use the diagram to determine the HOMO and the LUMO.

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following transition states are forbidden? 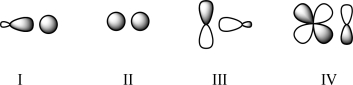
A)IV only
B)I and III
C)III only
D)III and IV
E)II and IV

A)IV only
B)I and III
C)III only
D)III and IV
E)II and IV

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following would be expected to contribute the HOMO to a transition-state complex? 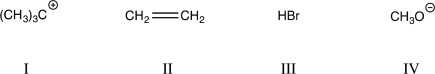
A)I only
B)I and III
C)I and II
D)II and III
E)II and IV
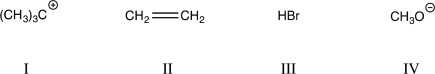
A)I only
B)I and III
C)I and II
D)II and III
E)II and IV

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
17
Briefly explain what determines whether a particular transition state is "allowed" or "forbidden."

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck


