Deck 13: Electrophilic Addition to Nonpolar Π Bonds 1: Addition of a Brønsted Acid
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/50
Play
Full screen (f)
Deck 13: Electrophilic Addition to Nonpolar Π Bonds 1: Addition of a Brønsted Acid
1
Which of the following alkenes would be susceptible to a carbocation rearrangement after the electrophilic addition step when treated with HBr?
A)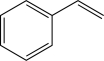
B)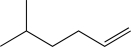
C)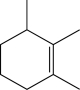
D)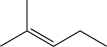
E)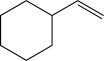
A)

B)

C)

D)

E)


2
Identify the reagent(s)needed to carry out the reaction below. 
A)H2O
B)HBr
C)H2O / H2SO4
D)H2SO4
E)CH3OH / H2SO4

A)H2O
B)HBr
C)H2O / H2SO4
D)H2SO4
E)CH3OH / H2SO4
H2O / H2SO4
3
Which of the following would not produce a racemic mixture of enantiomers when treated with HBr?
A)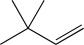
B)
C)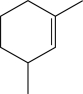
D)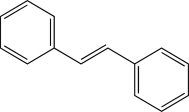
E)
A)

B)

C)

D)

E)


4
What is the rate-determining step in the reaction shown below? 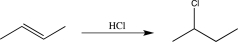
A)Nucleophilic addition
B)Coordination
C)Proton transfer
D)Electrophilic addition
E)Bimolecular nucleophilic substitution

A)Nucleophilic addition
B)Coordination
C)Proton transfer
D)Electrophilic addition
E)Bimolecular nucleophilic substitution

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
5
How many possible stereoisomers could be produced in the reaction below? 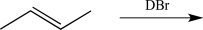
A)One
B)Two
C)Three
D)Four
E)Six
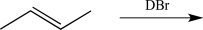
A)One
B)Two
C)Three
D)Four
E)Six

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is an intermediate in the reaction shown below? 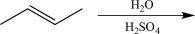
A)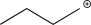
B)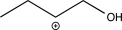
C)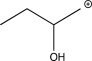
D)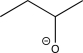
E)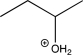
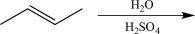
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following alkenes would produce the molecule below when treated with DCl? 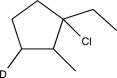
A)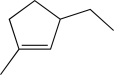
B)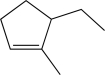
C)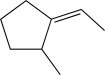
D)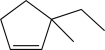
E)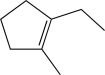

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
8
Predict the major product of the reaction below. 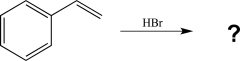
A)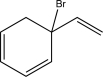
B)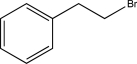
C)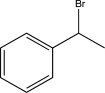
D)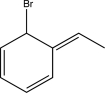
E)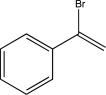

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following is unlikely to undergo an electrophilic addition reaction?
A)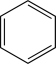
B)
C)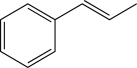
D)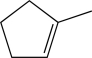
E)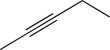
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
10
What will be the major product of the reaction below? 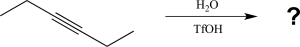
A)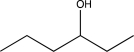
B)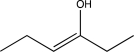
C)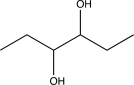
D)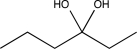
E)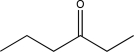
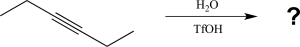
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following compounds would not produce the same major product as the others when treated with HCl? 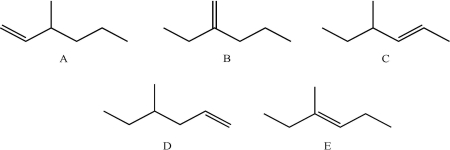
A)A
B)B
C)C
D)D
E)E

A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following contains a nonpolar π bond likely to participate in an electrophilic addition reaction?
A)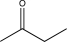
B)
C)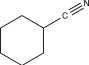
D)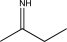
E)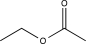
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
13
Which starting material would produce the mixture of products shown below? 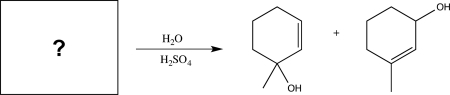
A)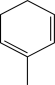
B)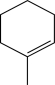
C)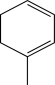
D)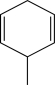
E)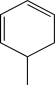

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
14
Predict the major product of the reaction below. 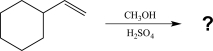
A)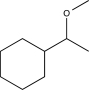
B)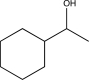
C)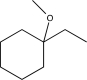
D)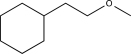
E)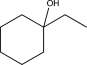
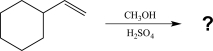
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
15
Identify the two elementary steps (in order)in the mechanism below. 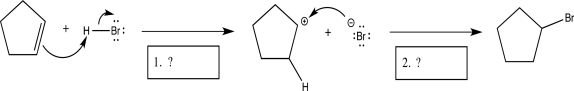
A)Electrophilic addition, proton transfer
B)Electrophilic elimination, coordination
C)Nucleophilic addition, electrophilic addition
D)Coordination, nucleophilic addition
E)Electrophilic addition, coordination

A)Electrophilic addition, proton transfer
B)Electrophilic elimination, coordination
C)Nucleophilic addition, electrophilic addition
D)Coordination, nucleophilic addition
E)Electrophilic addition, coordination

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
16
Identify the electron-poor species that participates in the electrophilic addition step in the reaction below. 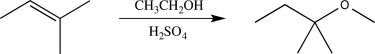
A)H2SO4
B)CH3CH2OH2+
C)H3O+
D)CH3CH2OH
E)H3SO4+

A)H2SO4
B)CH3CH2OH2+
C)H3O+
D)CH3CH2OH
E)H3SO4+

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following would yield a mixture of constitutional isomers as products when treated with HBr?
A)
B)
C)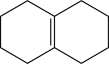
D)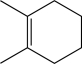
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
18
The electrophilic addition step in the reaction below can result in three carbocation intermediates.Rank these carbocation intermediates in order of increasing stability. 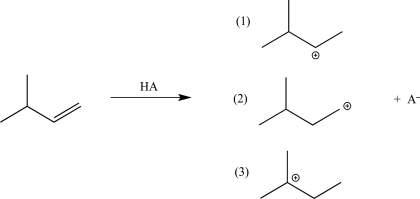
A)2 < 3 < 1
B)2 < 1 < 3
C)1 < 2 < 3
D)3 < 2 < 1
E)1 < 3 < 2

A)2 < 3 < 1
B)2 < 1 < 3
C)1 < 2 < 3
D)3 < 2 < 1
E)1 < 3 < 2

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
19
Predict the major product of the following reaction. 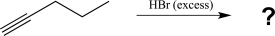
A)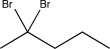
B)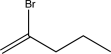
C)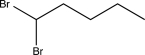
D)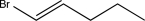
E)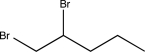
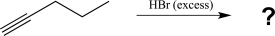
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
20
What is the most stable carbocation intermediate that can be formed in the reaction below? 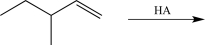
A)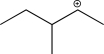
B)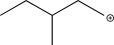
C)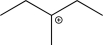
D)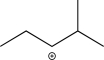
E)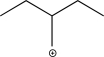

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
21
Predict the major product of the following reaction and draw the mechanism for the reaction. 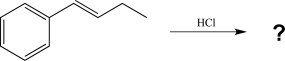


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
22
Briefly explain why the hypothetical reaction below does not occur. 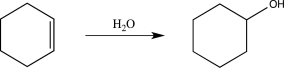


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
23
Label the electron-rich and electron-poor species in the elementary step below,and draw arrows to complete the mechanism. 


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
24
When the following molecule is treated with acid,it undergoes a reaction yielding a cyclic ether with the molecular formula C6H12O.Determine the structure of this product,and draw a mechanism to account for its formation. 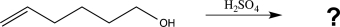
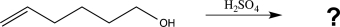

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
25
What would be the major product of the following reaction sequence? 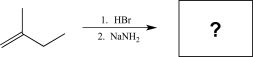
A)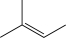
B)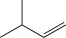
C)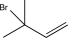
D)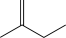
E)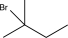

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
26
Draw a complete,detailed mechanism for the reaction below,and determine the structure of the product. 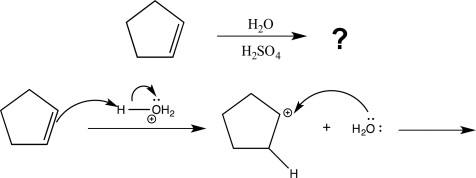


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
27
Draw a complete,detailed mechanism for the reaction below.Label the electron-rich and electron-poor sites in each step. 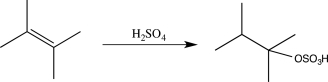


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following,when treated with HBr,would result in the formation of the same major product at both low and high temperatures?
A)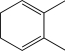
B)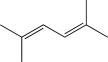
C)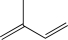
D)
E)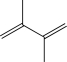
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
29
Give an example of a compound containing a nonpolar π bond that would be likely to participate in an electrophilic addition reaction.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
30
Draw three different alkenes that would produce the compound below when treated with HBr-one alkene that would undergo a 1,2-hydride shift,one that would undergo a 1,2-methyl shift,and one that would not undergo any carbocation rearrangements.Indicate which one falls into each category. 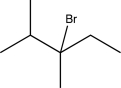


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
31
Briefly explain why the hypothetical reaction shown below is unlikely to occur. 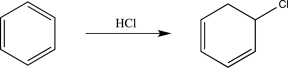


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
32
Predict the major product of the following reaction sequence. 
A)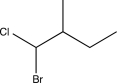
B)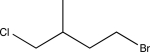
C)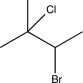
D)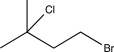
E)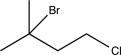

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
33
What would be the expected major product of the reaction below at a low temperature? 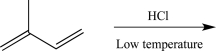
A)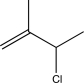
B)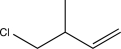
C)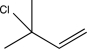
D)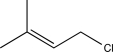
E)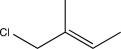
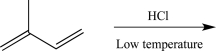
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
34
Draw the complete,detailed mechanism for the reaction below,and predict the product.Label each elementary step. 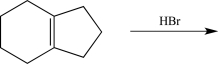


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following is true regarding the addition of a strong acid to a conjugated diene?
A)The major product formed at low temperatures is the most stable product.
B)The major product formed at high temperatures is the kinetic product.
C)The product of a 1,4-addition is always more stable than the product of a 1,2-addition.
D)The major product formed under thermodynamic control is the most stable product.
E)The major product formed the most rapidly is also the most stable product.
A)The major product formed at low temperatures is the most stable product.
B)The major product formed at high temperatures is the kinetic product.
C)The product of a 1,4-addition is always more stable than the product of a 1,2-addition.
D)The major product formed under thermodynamic control is the most stable product.
E)The major product formed the most rapidly is also the most stable product.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
36
Draw all possible products that could be formed in the reaction below,including stereoisomers. 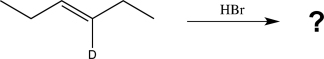


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
37
Show how the following compound could be synthesized from two different alkenes. 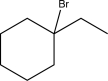


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
38
Give an example of a compound containing a nonpolar π bond that would be unlikely to participate in an electrophilic addition reaction.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
39
Predict the major product of the reaction below,and draw the mechanism for its formation. 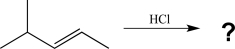


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
40
Draw the most stable carbocation intermediate formed after the electrophilic addition step in the reaction below. 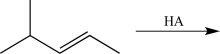


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
41
Show how you could carry out the following transformation,using more than one reaction if necessary. 


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
42
Fill in the missing starting material needed to complete the reaction below. 


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
43
Draw the major product that would be obtained if the reaction below were run at a high temperature,and draw the mechanism for its formation. 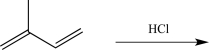
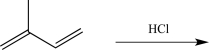

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
44
Draw the enol intermediate that would be formed in the reaction below. 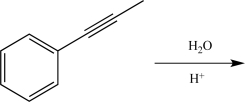


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
45
Draw a complete,detailed mechanism for the reaction below,and predict the major product. 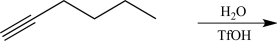


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
46
Draw the major 1,2-addition product and the major 1,4-addition product of the following reaction,and label each. 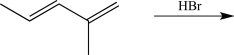


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
47
Choose the more stable carbocation intermediate formed in the following step,and explain why it is more stable. 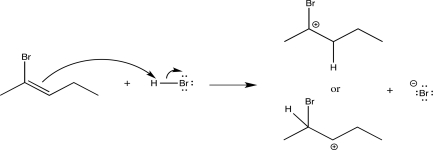


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
48
Show how the compound below can be synthesized from two different alkenes. 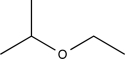


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
49
Label the thermodynamic product and the kinetic product formed in the reaction below. 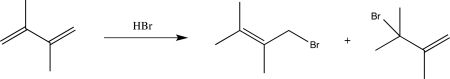


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
50
When certain compounds are treated with HCl,the resulting thermodynamic and kinetic products are identical.Give an example of such a compound.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck


