Deck 14: Electrophilic Addition to Nonpolar Π Bonds 2: Reactions Involving Cyclic Transition States
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/55
Play
Full screen (f)
Deck 14: Electrophilic Addition to Nonpolar Π Bonds 2: Reactions Involving Cyclic Transition States
1
What reagent is required to complete the following reaction? 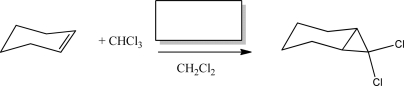
A)CH2N2
B)NaOH
C)HCl
D)PCl3
E)Cl2

A)CH2N2
B)NaOH
C)HCl
D)PCl3
E)Cl2
NaOH
2
When a carbene reacts with an alkene,what functionality is created?
A)Cyclopropane ring
B)Oxetane
C)Epoxide
D)Alkyne
E)Alcohol
A)Cyclopropane ring
B)Oxetane
C)Epoxide
D)Alkyne
E)Alcohol
Cyclopropane ring
3
Carbenes react with alkenes utilizing which fundamental step?
A)Nucleophilic addition
B)Electrophilic addition
C)Nucleophile elimination
D)Electrophile elimination
E)SN2
A)Nucleophilic addition
B)Electrophilic addition
C)Nucleophile elimination
D)Electrophile elimination
E)SN2
Electrophilic addition
4
What is the product of the following reaction? 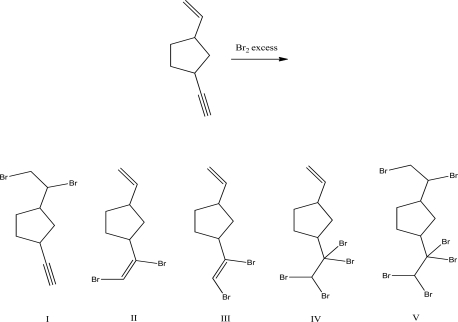
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
5
What is the product of the following reaction? 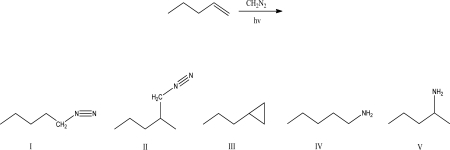
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
6
Carbenes are routinely created using alkenes plus which of the following molecules?
A)CH2N2
B)CH2Cl2
C)CH2Br2
D)All of the above (a-c)
E)None of the above
A)CH2N2
B)CH2Cl2
C)CH2Br2
D)All of the above (a-c)
E)None of the above

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
7
Which fundamental step can be found in the mechanism for the following reaction? 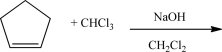
A)Homolysis
B)Heterolysis
C)Electrophilic elimination
D)Nucleophilic addition
E)Carbocation rearrangement
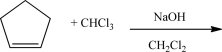
A)Homolysis
B)Heterolysis
C)Electrophilic elimination
D)Nucleophilic addition
E)Carbocation rearrangement

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is true about using oxymercuration-reduction to add an OH to an alkene?
A)The mechanism is susceptible to carbocation rearrangements.
B)The mechanism proceeds through a mercurinium ion intermediate.
C)Only terminal alkenes can be used as starting materials.
D)The mechanism proceeds using anti-Markovnikov addition.
E)Reduction with NaBH4 is stereoselective.
A)The mechanism is susceptible to carbocation rearrangements.
B)The mechanism proceeds through a mercurinium ion intermediate.
C)Only terminal alkenes can be used as starting materials.
D)The mechanism proceeds using anti-Markovnikov addition.
E)Reduction with NaBH4 is stereoselective.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
9
What is the product of the following reaction? 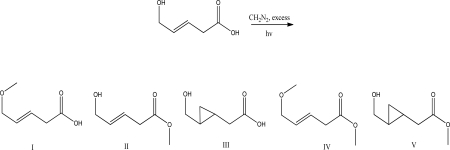
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is true regarding carbenes?
A)They are unreactive in halogenated solvents.
B)They can be used to create epoxides.
C)They are charged species.
D)They need to be generated in situ.
E)They are highly nucleophilic.
A)They are unreactive in halogenated solvents.
B)They can be used to create epoxides.
C)They are charged species.
D)They need to be generated in situ.
E)They are highly nucleophilic.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
11
What is the product of the following reaction? 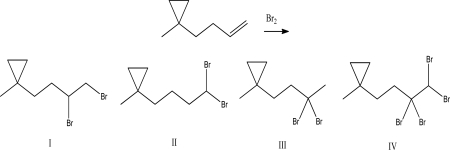
A)I
B)II
C)III
D)IV
E)A mixture of II and III

A)I
B)II
C)III
D)IV
E)A mixture of II and III

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
12
What is the product of the following reaction? 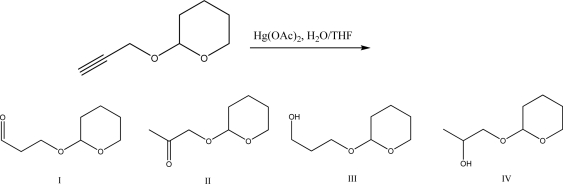
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
13
What is the product of the following reaction? 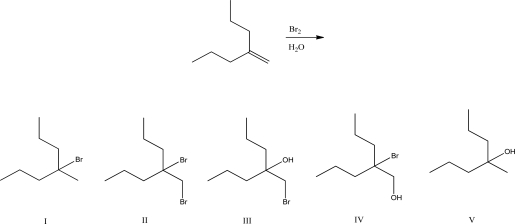
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
14
What reagent(s)will complete the following reaction? 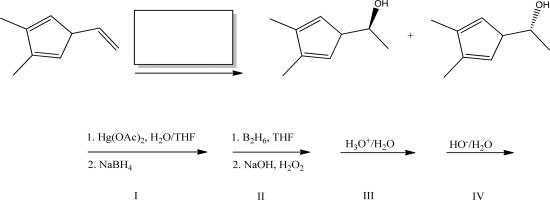
A)I
B)II
C)III
D)IV
E)Both III and IV will work.

A)I
B)II
C)III
D)IV
E)Both III and IV will work.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
15
When the alkene below is treated with Br2 and H2O,which is true about the stereochemistry of the product? 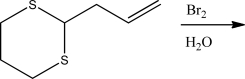
A)A single enantiomer will be produced.
B)A racemic mixture will result.
C)A mixture of diastereomers will be produced.
D)A single diastereomer will be produced.
E)A meso compound will be produced.

A)A single enantiomer will be produced.
B)A racemic mixture will result.
C)A mixture of diastereomers will be produced.
D)A single diastereomer will be produced.
E)A meso compound will be produced.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
16
What is the product of the following reaction? 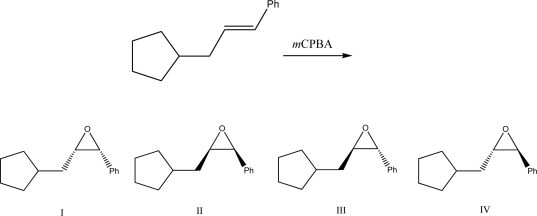
A)I
B)II
C)III
D)IV
E)A mixture of III and IV

A)I
B)II
C)III
D)IV
E)A mixture of III and IV

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
17
When an alkene is treated with Cl2,which of the following is not a part of the mechanism?
A)An electrophilic addition step.
B)An SN2 step.
C)The creation of a product with two anti Cl atoms.
D)A homolysis step.
E)The creation of a bromonium ion intermediate.
A)An electrophilic addition step.
B)An SN2 step.
C)The creation of a product with two anti Cl atoms.
D)A homolysis step.
E)The creation of a bromonium ion intermediate.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
18
When the alkene below is treated with Br2,which is true about the stereochemistry of the product? 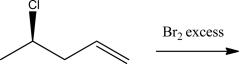
A)A pair of enantiomers will be produced.
B)A single diastereomer will be produced.
C)A mixture of diastereomers will be produced.
D)A meso compound will be produced.
E)The product will be achiral.

A)A pair of enantiomers will be produced.
B)A single diastereomer will be produced.
C)A mixture of diastereomers will be produced.
D)A meso compound will be produced.
E)The product will be achiral.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
19
What starting material is required to complete the following transformation? 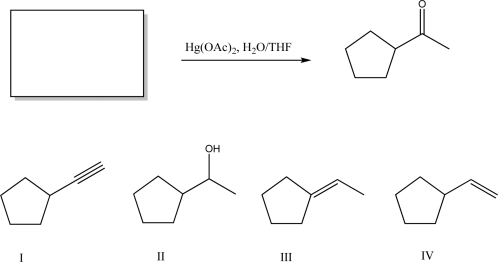
A)I
B)II
C)III
D)IV
E)Both III and IV are acceptable starting materials.

A)I
B)II
C)III
D)IV
E)Both III and IV are acceptable starting materials.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
20
What is the product of the following reaction? 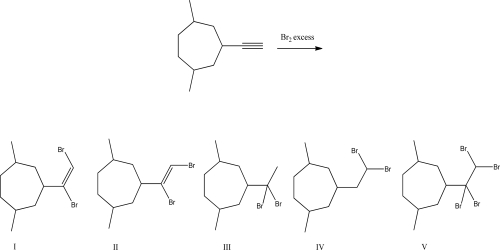
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
21
Draw the detailed mechanism and the product for the following reaction. 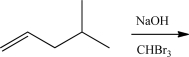


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
22
What reagent is required to complete the following reaction? 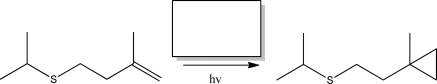


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
23
What reagent(s)will complete the following reaction? 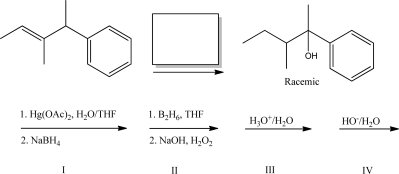
A)I
B)II
C)III
D)IV
E)Both I and III will work.

A)I
B)II
C)III
D)IV
E)Both I and III will work.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
24
What is the product of the following reaction? 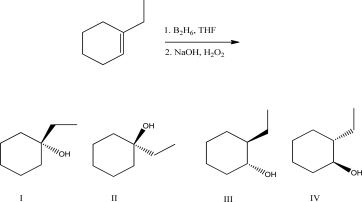
A)I
B)II
C)III
D)IV
E)A mixture of III and IV

A)I
B)II
C)III
D)IV
E)A mixture of III and IV

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
25
What is the product of the following reaction? 


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
26
Identify the reagent(s)required to synthesize the following racemic halohydrin. 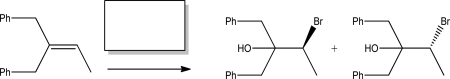


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
27
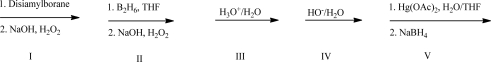
What reagent(s)will complete the following reaction? 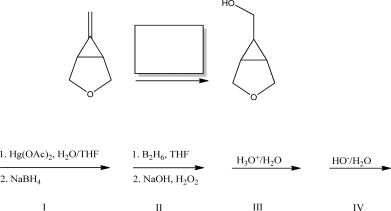
A)I
B)II
C)III
D)IV
E)Both I and III will work.

A)I
B)II
C)III
D)IV
E)Both I and III will work.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
28
The following alkene is treated with Br2.What is the stereochemical relationship of the two bromine atoms in the product? 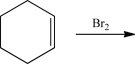


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
29
What is the formal charge on a carbene?

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
30
What reagent best completes the following reaction? 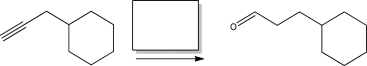
A)I
B)II
C)III
D)IV
E)V


A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following reactions will produce the highest yield? 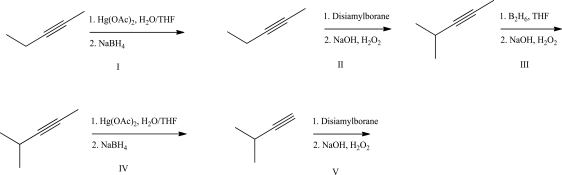
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
32
What is the product of the following set of reactions? 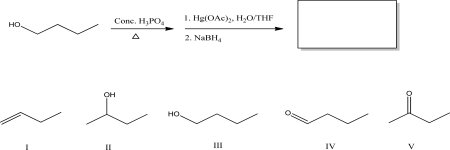
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
33
What is the product of the following reaction? 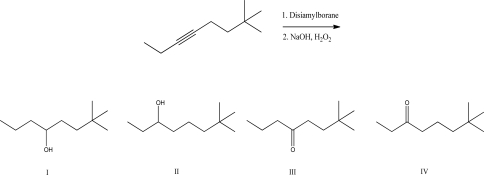
A)I
B)II
C)III
D)IV
E)A mixture of III and IV

A)I
B)II
C)III
D)IV
E)A mixture of III and IV

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
34
Draw the detailed mechanism and the product for the following reaction.Be sure to include stereochemistry,if applicable. 


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
35
What is the product of the following reaction? 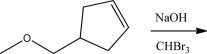


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
36
Show how the following molecule can be synthesized from an alkene. 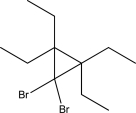


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
37
What is the product of the following reaction? 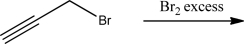
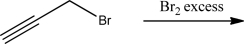

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
38
What is the product of the following reaction? 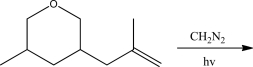


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
39
A carbon atom that possesses two bonds and a lone pair of electrons is called a ________.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
40
Which fundamental step does not occur in the mechanism for the reaction below? 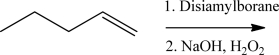
A)Nucleophilic addition
B)Nucleophile elimination
C)Heterolysis
D)Coordination
E)Proton transfer
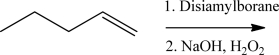
A)Nucleophilic addition
B)Nucleophile elimination
C)Heterolysis
D)Coordination
E)Proton transfer

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
41
Draw the product for the following reaction. 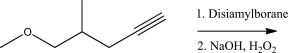


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
42
Give two ways to synthesize an epoxide starting with the given alkene.Which one is more efficient? Explain. 


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
43
Draw the correct starting material for the reaction below. 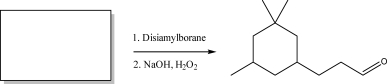


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
44
What is the product of the following reaction? 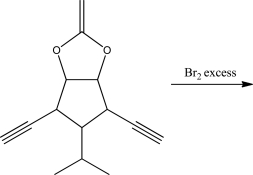


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
45
Show the reagent(s)required to complete the following reaction. 


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
46
Supply the three missing products. 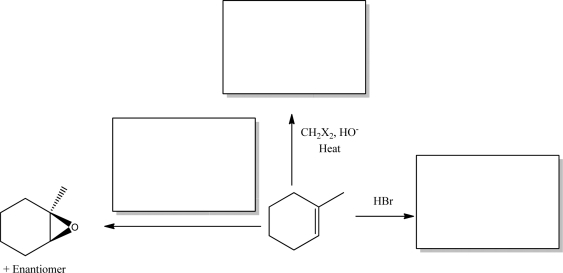


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
47
Identify missing compounds A,B,and C. 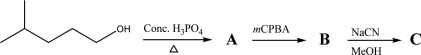


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
48
Draw the alkene that can be epoxidized using mCPBA to yield the following compound. 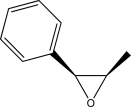


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
49
Draw the product of the following reaction. 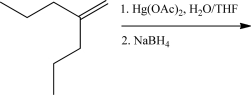


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
50
Provide the reagents needed to complete the following reaction. 


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
51
What is the product of the following reaction. 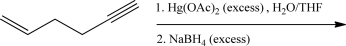
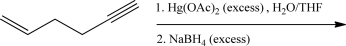

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
52
What is the product of the following reaction? 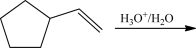


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
53
Draw the products of the following reactions. 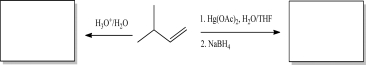


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
54
Show how the following compound can be synthesized from an alkene. 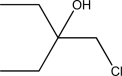


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
55
Name the reagent(s)required to complete the following reaction. 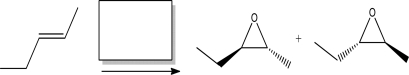


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck


