Deck 21: Organic Synthesis 2: Intermediate Topics in Synthesis Design,and Useful Reduction and Oxidation Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/50
Play
Full screen (f)
Deck 21: Organic Synthesis 2: Intermediate Topics in Synthesis Design,and Useful Reduction and Oxidation Reactions
1
What is the starting material for the following reaction? 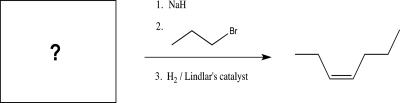
A)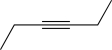
B)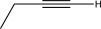
C)
D)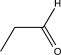
E)

A)

B)
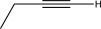
C)

D)

E)

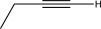
2
What is the product of the following reaction sequence? 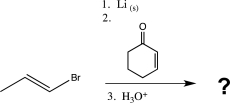
A)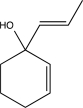
B)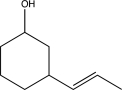
C)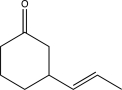
D)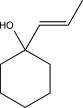
E)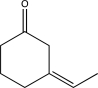

A)

B)

C)

D)

E)


3
Which of the following is not part of the mechanism for the catalytic hydrogenation of an alkene?
A)Adsorption of hydrogen gas onto metal surface.
B)Formation of CH bond and liberation of C atom from surface.
C)Adsorption of alkene onto metal surface.
D)Proton transfer from metal to carbon.
E)Liberation of alkane from surface.
A)Adsorption of hydrogen gas onto metal surface.
B)Formation of CH bond and liberation of C atom from surface.
C)Adsorption of alkene onto metal surface.
D)Proton transfer from metal to carbon.
E)Liberation of alkane from surface.
Proton transfer from metal to carbon.
4
Which of the following steps involves umpolung?
A)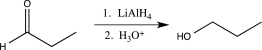
B)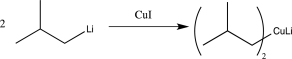
C)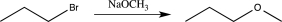
D)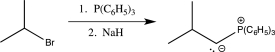
E)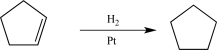
A)
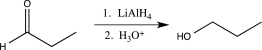
B)

C)
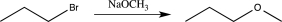
D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
5
Predict the major product of the following reaction (assume one equivalent of H2 is present). 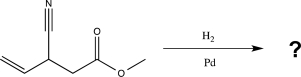
A)
B)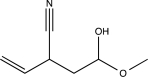
C)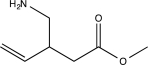
D)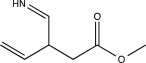
E)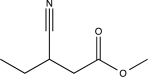

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following reactions would not function as an effective protection reaction for an alcohol?
A)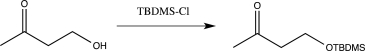
B)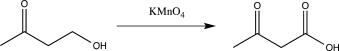
C)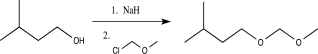
D)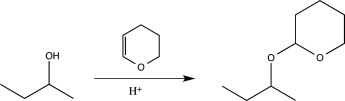
E)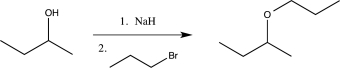
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
7
What is the major product of the following reaction? 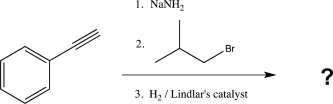
A)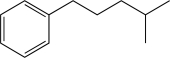
B)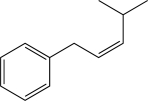
C)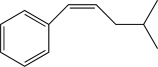
D)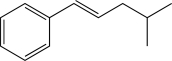
E)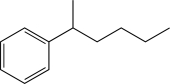

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following reagents would be the best choice to carry out the synthetic step shown below? 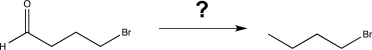
A)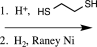
B)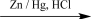
C)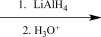
D)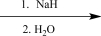
E)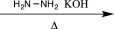
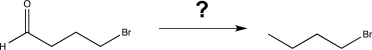
A)

B)
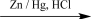
C)
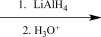
D)
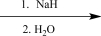
E)
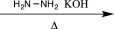

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
9
In which of the following reactions does a reversal of polarity at a carbon atom take place?
A)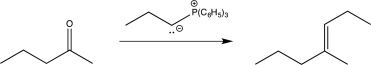
B)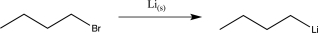
C)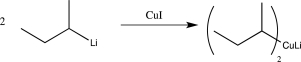
D)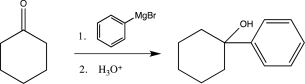
E)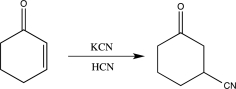
A)

B)
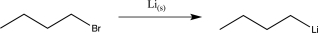
C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
10
Fill in the starting material for the following reaction. 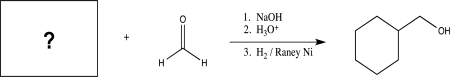
A)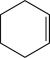
B)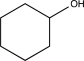
C)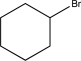
D)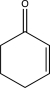
E)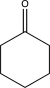

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
11
Predict the product of the following reaction. 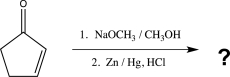
A)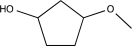
B)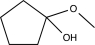
C)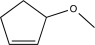
D)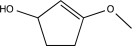
E)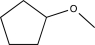

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following transformations would require the use of a protecting group? (Reagents are not shown.)
A)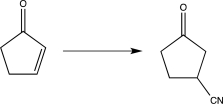
B)
C)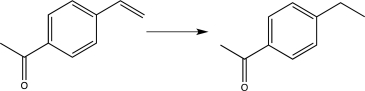
D)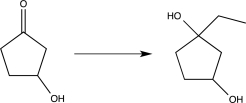
E)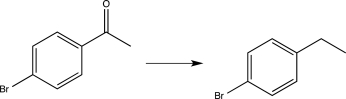
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
13
What is the missing starting material for the synthesis shown below? 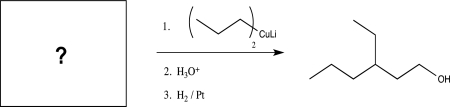
A)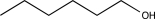
B)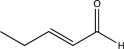
C)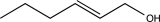
D)
E)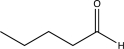

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
14
What is the major product of the following reaction? 
A)
B)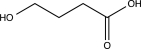
C)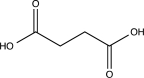
D)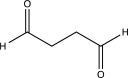
E)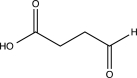

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
15
Which mechanism step is involved in the protection reaction shown below? 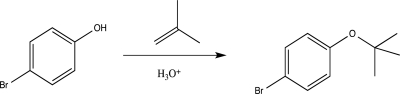
A)Bimolecular elimination
B)Carbocation rearrangement
C)Electrophilic addition
D)Bimolecular nucleophilic substitution
E)Nucleophilic addition

A)Bimolecular elimination
B)Carbocation rearrangement
C)Electrophilic addition
D)Bimolecular nucleophilic substitution
E)Nucleophilic addition

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following reaction sequences could be used to synthesize the molecule shown below? 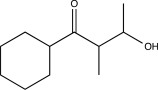
A)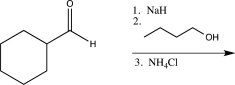
B)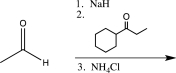
C)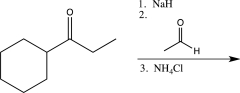
D)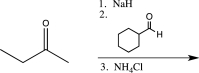
E)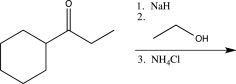

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following alkyl halides will not form a Grignard reagent when reacted with magnesium? 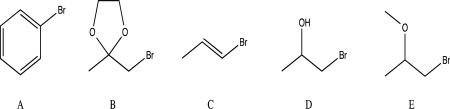
A)A
B)B
C)C
D)D
E)E

A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
18
Predict the major product of the following reaction (assume one equivalent of H2 is present). 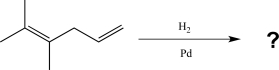
A)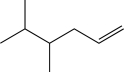
B)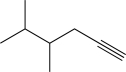
C)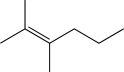
D)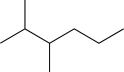
E)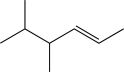

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following reactions does not utilize a selective reagent?
A)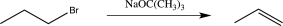
B)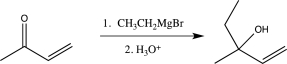
C)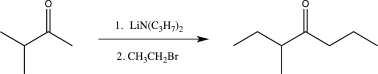
D)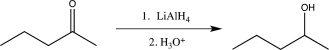
E)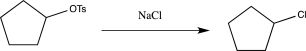
A)
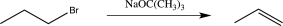
B)
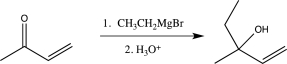
C)

D)
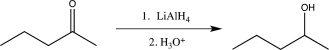
E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
20
Predict the product of the following reaction sequence. 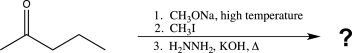
A)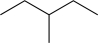
B)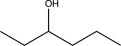
C)
D)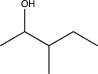
E)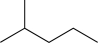
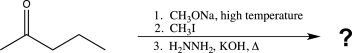
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
21
Show how you would synthesize the molecule below using propionaldehyde as the only source
of carbon present in the final product.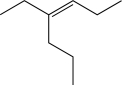
of carbon present in the final product.


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
22
What is the expected product of the reaction below? 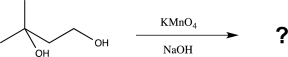
A)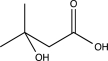
B)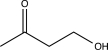
C)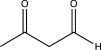
D)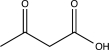
E)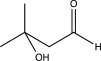
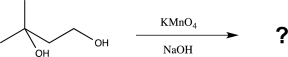
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
23
Fill in the missing reagent to carry out the following synthetic step. 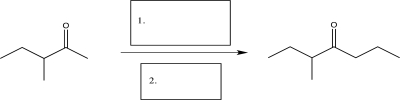


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
24
Propose a way to carry out the following transformation beginning with the starting material shown and using any other reagents necessary. 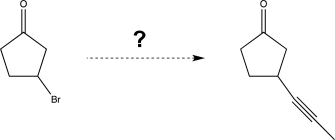


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
25
Determine the product of the following reaction sequence. 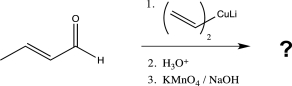
A)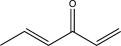
B)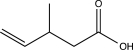
C)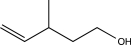
D)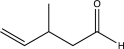
E)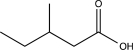

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
26
A student proposes the following reaction.Explain the issue that this student may encounter,and suggest an alternative route to achieve the same synthetic transformation. 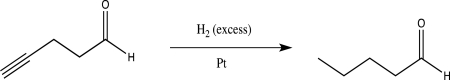


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
27
Show how you would carry out the following transformation,using any reagents necessary (requires more than one step). 


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
28
Show how to convert the starting material at the left to the desired product at the right. 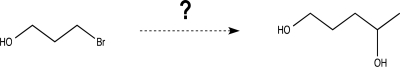


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
29
Draw a complete,detailed mechanism for the protection step shown below,and predict the product. 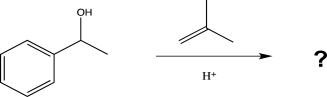


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
30
Briefly explain why the following reaction would be a poor choice as a way to protect the aldehyde. 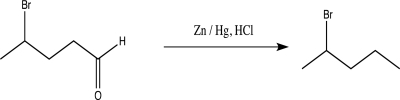


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
31
Show how you would carry out the following transformation,using any reagents necessary. 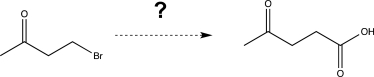


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
32
Fill in the boxes with a starting material and reagents to complete the synthetic scheme below. 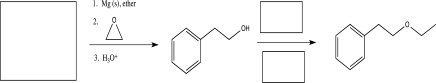


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
33
Which would be the best reagent to carry out the reaction shown below? 
A)KMnO4/NaOH
B)H2CrO4/H2SO4
C)Zn/Hg, HCl
D)NaBH4/H2O
E)PCC

A)KMnO4/NaOH
B)H2CrO4/H2SO4
C)Zn/Hg, HCl
D)NaBH4/H2O
E)PCC

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
34
Using the molecule shown at the left as your only source of carbon,propose a synthesis of the product shown at the right. 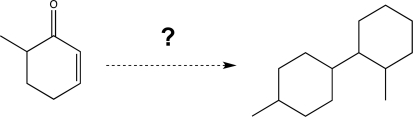


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
35
What is the product of the following reaction? 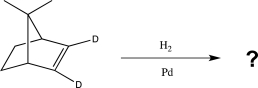
A)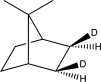
B)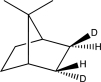
C)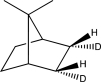
D)
E)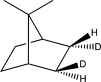

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
36
Explain why the following reaction would be unsuccessful,and suggest a better alternative synthetic route. 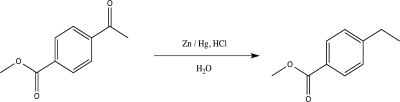


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following alcohols cannot be oxidized by H2CrO4?
A)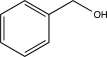
B)
C)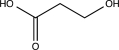
D)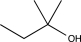
E)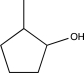
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
38
Show how the following molecule could be synthesized from an α,β-unsaturated ketone or aldehyde. 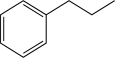


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
39
Give an example of an umpolung reaction,and indicate the carbon atom at which polarity reversal is occurring.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
40
A student attempted the following synthetic step but did not obtain the desired major product.Explain why the reaction was unsuccessful,and suggest a better synthetic route. 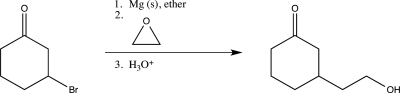


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
41
Propose a synthetic route to carry out the transformation shown below. 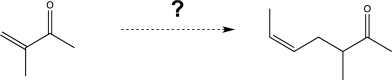


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
42
Propose a synthesis of the following molecule using benzaldehyde as your only source of carbon.Additional inorganic reagents may be utilized. 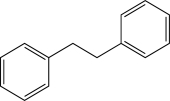


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
43
Predict the major product of the following reaction. 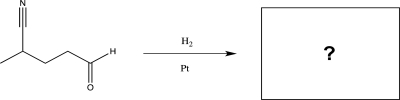


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
44
Fill in the reagent needed to carry out the following reaction. 


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
45
Fill in the reagent needed to carry out the following reaction. 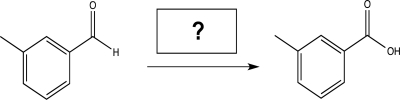


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
46
Fill in the missing reagents and intermediates to complete the synthetic scheme below. 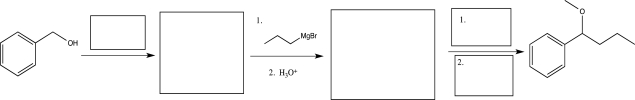


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
47
Design a synthesis of the following molecule using organic precursors with no more than six carbons.Additional inorganic reagents may be utilized. 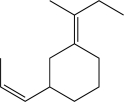


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
48
Propose a synthesis of the following molecule using reagents with five or fewer carbon atoms. 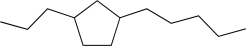


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
49
Propose a synthesis of the product at the right using the given starting material and any other reagents necessary. 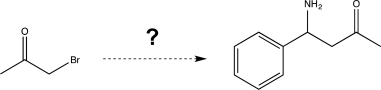


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
50
Propose a synthesis of the following molecule using butyraldehyde as your only source of carbon. 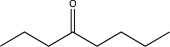


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck


