Deck 12: Energy and Hydrocarbons
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/57
Play
Full screen (f)
Deck 12: Energy and Hydrocarbons
1
A compound containing seven carbon atoms could be
A) heptane.
B) octane.
C) hexane.
D) nonane.
A) heptane.
B) octane.
C) hexane.
D) nonane.
heptane.
2
The name of the C2H5- group is
A) methyl
B) ethyl.
C) propyl.
D) butyl.
A) methyl
B) ethyl.
C) propyl.
D) butyl.
ethyl.
3
Which of the following will not improve the octane rating of a gasoline?
A) increasing the percentage of branched-chain hydrocarbons
B) increasing the percentage of aromatic hydrocarbons
C) increasing the percentage of straight-chain hydrocarbons
D) adding octane enhancers
A) increasing the percentage of branched-chain hydrocarbons
B) increasing the percentage of aromatic hydrocarbons
C) increasing the percentage of straight-chain hydrocarbons
D) adding octane enhancers
increasing the percentage of straight-chain hydrocarbons
4
Which process is used to increase the amount of the gasoline fraction in refining to match commercial demand?
A) reforming
B) oxygenating
C) cracking
D) gasification
A) reforming
B) oxygenating
C) cracking
D) gasification

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
5
Fermentation of carbohydrates is a source of
A) MTBE.
B) ethanol.
C) ethers.
D) methanol.
A) MTBE.
B) ethanol.
C) ethers.
D) methanol.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
6
The hydrocarbon represented by the following model would be called 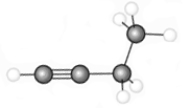
A) unsaturated.
B) aromatic.
C) an alkene.
D) all of the above

A) unsaturated.
B) aromatic.
C) an alkene.
D) all of the above

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
7
Which is a characteristic feature of alkenes?
A) general formula of CnH2n + 2
B) tetrahedral geometry
C) unsaturated
D) hydrocarbons which contain only single bonds
A) general formula of CnH2n + 2
B) tetrahedral geometry
C) unsaturated
D) hydrocarbons which contain only single bonds

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
8
The symbol shown below.represents 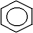
A) a cycloalkane.
B) cyclohexane.
C) hexane.
D) benzene.

A) a cycloalkane.
B) cyclohexane.
C) hexane.
D) benzene.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
9
All combustion reactions of fossil fuels
A) give off energy.
B) are complete reactions with oxygen.
C) are endothermic reactions.
D) are l00% efficient.
A) give off energy.
B) are complete reactions with oxygen.
C) are endothermic reactions.
D) are l00% efficient.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
10
Which is a representation of cyclohexane?
A)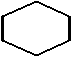
B) C6H6
C)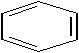
D)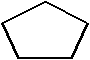
A)

B) C6H6
C)

D)


Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
11
Coal gasification is a process for making
A) synthesis gas.
B) molecules that are precursors of organic chemicals.
C) methane.
D) all of these
A) synthesis gas.
B) molecules that are precursors of organic chemicals.
C) methane.
D) all of these

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
12
What is the bond angle around a triple bond?
A) 90
B) l09.5
C) l20
D) l80
A) 90
B) l09.5
C) l20
D) l80

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
13
Which alcohol is called wood alcohol?
A) ethanol
B) isopropyl alcohol
C) 1-propanol
D) methanol
A) ethanol
B) isopropyl alcohol
C) 1-propanol
D) methanol

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is ethylene?
A) CH2=CHCH3
B) CH2=CH2
C) H-C=C-H
D) CH3-CH3
A) CH2=CHCH3
B) CH2=CH2
C) H-C=C-H
D) CH3-CH3

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
15
The complete combustion of C2H6 yields
A) C2H4 and H2.
B) CO and H2O.
C) CO2 and H2O.
D) C and H2.
A) C2H4 and H2.
B) CO and H2O.
C) CO2 and H2O.
D) C and H2.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
16
Butane [CH3CH2CH2CH3] and 2-methylpropane [(CH3)2CHCH3] are
A) stereoisomers.
B) cis-trans isomers.
C) structural isomers.
D) optical isomers.
A) stereoisomers.
B) cis-trans isomers.
C) structural isomers.
D) optical isomers.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
17
At current usage rates, world reserves of which fuel below are estimated to last for the least number of years?
A) natural gas
B) hydrogen
C) petroleum
D) coal
A) natural gas
B) hydrogen
C) petroleum
D) coal

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
18
Which is a characteristic of alkanes?
A) contain C=C
B) general formula CnH2n
C) saturated
D) cis-trans isomers
A) contain C=C
B) general formula CnH2n
C) saturated
D) cis-trans isomers

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
19
Which fossil fuel furnishes the most heat energy per gram?
A) petroleum
B) charcoal
C) natural gas
D) coal
A) petroleum
B) charcoal
C) natural gas
D) coal

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
20
Consider the following structure. 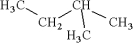 Which of the following is an isomer of this structure?
Which of the following is an isomer of this structure?
A)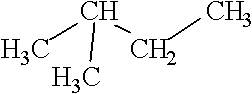
B)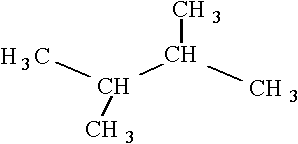
C)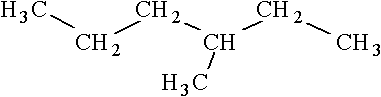
D)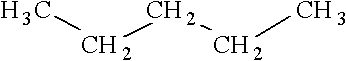
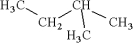 Which of the following is an isomer of this structure?
Which of the following is an isomer of this structure?A)

B)

C)

D)
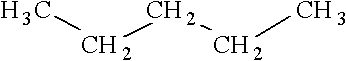

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
21
Which fossil fuel has a molecular structure containing cyclic hydrocarbons?
A) coal
B) liquid propane
C) natural gas
D) none of these
A) coal
B) liquid propane
C) natural gas
D) none of these

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
22
What are the bond angles around the carbon atoms in alkanes?
A) 90
B) l09.5
C) l20
D) l80
A) 90
B) l09.5
C) l20
D) l80

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
23
What term is used to describe the amount of heat energy released from burning?
A) heat of fusion
B) heat of vaporization
C) heat of combustion
D) heat of formation
A) heat of fusion
B) heat of vaporization
C) heat of combustion
D) heat of formation

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
24
The octane rating of straight run gasoline from fractional distillation of petroleum can be raised by
A) catalytic reforming.
B) adding tertiary-butyl alcohol.
C) adding ethanol.
D) all of the above
A) catalytic reforming.
B) adding tertiary-butyl alcohol.
C) adding ethanol.
D) all of the above

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
25
Which substance is grain alcohol?
A) methanol
B) ethanol
C) MTBE
D) benzene
A) methanol
B) ethanol
C) MTBE
D) benzene

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
26
Petroleum is separated into fractions in the refining process. Which of the following fractions has molecules with the largest size?
A) gas
B) kerosene
C) paraffin wax
D) straight-run gasoline
A) gas
B) kerosene
C) paraffin wax
D) straight-run gasoline

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
27
The fuel identified as M85 is
A) a substance with a molar mass of 85.
B) a fuel mixture of 85% methanol and 15% gasoline.
C) a fuel with a boiling point above 85 C.
D) a fuel that is 85% gasoline and 15% ethanol.
A) a substance with a molar mass of 85.
B) a fuel mixture of 85% methanol and 15% gasoline.
C) a fuel with a boiling point above 85 C.
D) a fuel that is 85% gasoline and 15% ethanol.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
28
Which fossil fuel burns cleaner and more completely?
A) coal
B) natural gas
C) petroleum
D) wood
A) coal
B) natural gas
C) petroleum
D) wood

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
29
Gasoline is a
A) single, simple hydrocarbon.
B) single, complex hydrocarbon.
C) mixture of C12-C16 hydrocarbons.
D) mixture of C5-C12 hydrocarbons.
A) single, simple hydrocarbon.
B) single, complex hydrocarbon.
C) mixture of C12-C16 hydrocarbons.
D) mixture of C5-C12 hydrocarbons.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following is not true about the compound MTBE?
A) it is an ether
B) made by fermentation
C) it is a carcinogen
D) used to oxygenate gasoline
A) it is an ether
B) made by fermentation
C) it is a carcinogen
D) used to oxygenate gasoline

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
31
Carcinogens are compounds that are
A) never used as fuels.
B) cancer causing agents.
C) not found in petroleum.
D) rarely aromatic.
A) never used as fuels.
B) cancer causing agents.
C) not found in petroleum.
D) rarely aromatic.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
32
Fractional distillation of petroleum
A) separates and purifies all of the hydrocarbons in crude oil.
B) separates fractions, each of which contain many hydrocarbons.
C) is the final step in refining gasoline.
D) fractions large molecules into smaller ones.
A) separates and purifies all of the hydrocarbons in crude oil.
B) separates fractions, each of which contain many hydrocarbons.
C) is the final step in refining gasoline.
D) fractions large molecules into smaller ones.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
33
The combustible products of most coal gasification techniques are
A) nitrogen, hydrogen, and carbon monoxide.
B) nitrogen and hydrogen.
C) hydrogen and carbon monoxide.
D) carbon dioxide and hydrogen.
A) nitrogen, hydrogen, and carbon monoxide.
B) nitrogen and hydrogen.
C) hydrogen and carbon monoxide.
D) carbon dioxide and hydrogen.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
34
Gasohol (E85) contains
A) 15% ethanol mixed with unleaded gasoline.
B) 10% methanol mixed with unleaded gasoline.
C) 85% ethanol mixed with unleaded gasoline.
D) three grams of tertiary-butyl alcohol per gallon.
A) 15% ethanol mixed with unleaded gasoline.
B) 10% methanol mixed with unleaded gasoline.
C) 85% ethanol mixed with unleaded gasoline.
D) three grams of tertiary-butyl alcohol per gallon.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following is not true about benzene?
A) has the formula C6H12
B) is a carcinogen
C) increases the octane rating of gasoline
D) is a cyclic hydrocarbon
A) has the formula C6H12
B) is a carcinogen
C) increases the octane rating of gasoline
D) is a cyclic hydrocarbon

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
36
Which fuel has the largest heat of combustion per gram?
A) hydrogen
B) ethanol
C) wood
D) gasoline
A) hydrogen
B) ethanol
C) wood
D) gasoline

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
37
The purpose of coal gasification is to
A) pressurize coal so the coal will be compact.
B) produce a relatively clean burning, gaseous fuel.
C) make more consumer products from coal.
D) put railroads out of business.
A) pressurize coal so the coal will be compact.
B) produce a relatively clean burning, gaseous fuel.
C) make more consumer products from coal.
D) put railroads out of business.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
38
Octane ratings are based on comparison of the fuel being tested with the knocking properties of mixtures of
A) hexane and heptane.
B) isooctane and hexane.
C) isooctane and heptane.
D) heptane and nonane.
A) hexane and heptane.
B) isooctane and hexane.
C) isooctane and heptane.
D) heptane and nonane.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following compounds would you expect to have the highest octane rating?
A) pentane, C5H12 , CH3CH2CH2CH2CH3
B) hexane, C6H14 , CH3CH2CH2CH2CH2CH3
C) isooctane, C8H18 , (CH3) 3CCH2CH(CH3) 2
D) heptane, C7H16 , CH3CH2CH2CH2CH2CH2CH3
A) pentane, C5H12 , CH3CH2CH2CH2CH3
B) hexane, C6H14 , CH3CH2CH2CH2CH2CH3
C) isooctane, C8H18 , (CH3) 3CCH2CH(CH3) 2
D) heptane, C7H16 , CH3CH2CH2CH2CH2CH2CH3

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
40
A coal gasification process involving a catalyst, carbon monoxide, hydrogen, steam and coal produces
A) only CO.
B) liquid petroleum.
C) octane.
D) methane and carbon dioxide.
A) only CO.
B) liquid petroleum.
C) octane.
D) methane and carbon dioxide.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
41
The structure below represents an aromatic hydrocarbon which is unsaturated and has a higher octane rating than hexane. 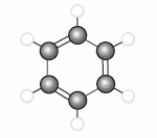


Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
42
Consider the following model of ethanol, CH3CH2OH. 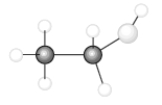 During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
The number of carbon to hydrogen single bonds that are broken is_______.
 During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.The number of carbon to hydrogen single bonds that are broken is_______.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
43
The following two hydrocarbon models represent structural isomers of each other. 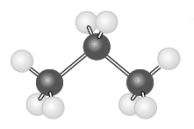
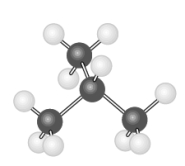



Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
44
Consider the following model of ethanol, CH3CH2OH.  During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
In the balanced equation for the combustion of one mole of ethanol, the coefficient of water is _____.
 During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.In the balanced equation for the combustion of one mole of ethanol, the coefficient of water is _____.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the bond types shown below gives rise to cis-trans isomers?
A) C - C
B) C - H
C)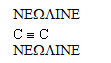
D) C -C
A) C - C
B) C - H
C)
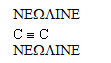
D) C -C

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
46
Identify the alkane shown below.
A) C5H12
B) C10H20
C) C3H4
D) C18H40
A) C5H12
B) C10H20
C) C3H4
D) C18H40

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
47
Consider the following model of ethanol, CH3CH2OH.  During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
Using the data in the following table,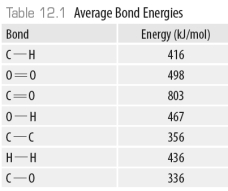 The amount of energy required to break all the C-H bonds in ethanol is ______kJ.
The amount of energy required to break all the C-H bonds in ethanol is ______kJ.
 During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.Using the data in the following table,
 The amount of energy required to break all the C-H bonds in ethanol is ______kJ.
The amount of energy required to break all the C-H bonds in ethanol is ______kJ.
Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
48
Consider the following model of ethanol, CH3CH2OH.  During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
Using the data in the following table,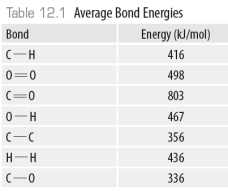 The amount of energy released in the formation of all the O-H bonds in the product water is ______kJ.
The amount of energy released in the formation of all the O-H bonds in the product water is ______kJ.
 During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.Using the data in the following table,
 The amount of energy released in the formation of all the O-H bonds in the product water is ______kJ.
The amount of energy released in the formation of all the O-H bonds in the product water is ______kJ.
Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the bond types shown below is the strongest? 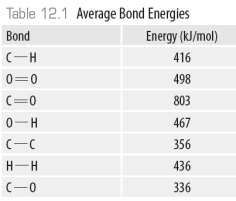
A) C - C
B) C = O
C) O - H
D) O = O

A) C - C
B) C = O
C) O - H
D) O = O

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
50
Which hydrocarbon would be isolated at the top of a fractionation column?
A) C5H12
B) C10H22
C) C3H8
D) C18H36
A) C5H12
B) C10H22
C) C3H8
D) C18H36

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
51
The following compound could represent the principle component of natural gas. 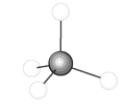


Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
52
Identify the alkene shown below.
A) C5H12
B) C10H20
C) C3H4
D) C18H40
A) C5H12
B) C10H20
C) C3H4
D) C18H40

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
53
The hydrocarbon represented by the model below has cis and trans isomers. 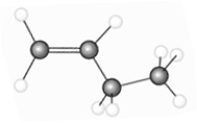


Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
54
The correct name of the following compound is 2-dimethylpentane. 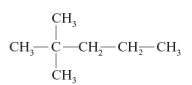


Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
55
The hydrocarbon model shown below could represent a saturated compound with the general formula CnH2n+2. 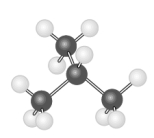


Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
56
Among the alkanes, alkenes, and alkynes only the alkanes contain only single bonds.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
57
Consider the following model of ethanol, CH3CH2OH.  During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
During the combustion of one mole of ethanol, the number of O-H bonds formed is _____.
 During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.During the combustion of one mole of ethanol, the number of O-H bonds formed is _____.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck


