Deck 2: The Chemical View of Matter
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/59
Play
Full screen (f)
Deck 2: The Chemical View of Matter
1
Alfred Nobel ____________?
A) discovered dynamite
B) proposed the metric system
C) developed the STM, scanning tunneling microscope
D) discovered kinetic energy
A) discovered dynamite
B) proposed the metric system
C) developed the STM, scanning tunneling microscope
D) discovered kinetic energy
discovered dynamite
2
Sublimation is a characteristic physical property of
A) chlorine (Cl2, liquid).
B) oxygen (O2, gas).
C) bromine (Br2, liquid).
D) iodine (I2, solid).
A) chlorine (Cl2, liquid).
B) oxygen (O2, gas).
C) bromine (Br2, liquid).
D) iodine (I2, solid).
iodine (I2, solid).
3
Which mixture is heterogeneous?
A) salt and water
B) water and oil
C) sweetened hot tea
D) Ivory soap bar
A) salt and water
B) water and oil
C) sweetened hot tea
D) Ivory soap bar
water and oil
4
Which of the following sets, is a list of the symbols for an element and a compound (in that order)?
A) Mg, CO
B) CO, CO2
C) CO, Co
D) H2O2, P
A) Mg, CO
B) CO, CO2
C) CO, Co
D) H2O2, P

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
5
Most samples of matter occur in nature as
A) elements.
B) compounds.
C) homogeneous samples.
D) mixtures.
A) elements.
B) compounds.
C) homogeneous samples.
D) mixtures.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
6
Which statement describes a physical property of oxygen?
A) Oxygen supports burning of gasoline.
B) Oxygen has a density of 0.0014 g/mL.
C) Oxygen is required for human metabolism of food.
D) Oxygen combines with iron causing the formation of rust.
A) Oxygen supports burning of gasoline.
B) Oxygen has a density of 0.0014 g/mL.
C) Oxygen is required for human metabolism of food.
D) Oxygen combines with iron causing the formation of rust.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is not one of the common states of matter?
A) solid
B) plasma
C) liquid
D) gas
A) solid
B) plasma
C) liquid
D) gas

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
8
What information is not provided by the formula, C4H10, for butane?
A) butane being an organic compound
B) the molecular formula
C) the relative number of atoms of each kind
D) the shape of the molecule
A) butane being an organic compound
B) the molecular formula
C) the relative number of atoms of each kind
D) the shape of the molecule

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following sets, is a list of the symbols that could represent the following substances, respectively? lead a compound of equal parts hydrogen and oxygen elemental oxygen
A) PB, H2O2, O
B) Pb, HO, O
C) Pb, H2O2, O2
D) PB, HO, O2
A) PB, H2O2, O
B) Pb, HO, O
C) Pb, H2O2, O2
D) PB, HO, O2

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
10
Which is not a mixture?
A) pure water
B) mayonnaise
C) strawberry Kool-Aid® drink
D) rock
A) pure water
B) mayonnaise
C) strawberry Kool-Aid® drink
D) rock

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
11
A process is probably a chemical reaction if
A) it produces light.
B) a solid appears when two solutions are mixed.
C) bubbles start to form when two substances are mixed.
D) all of these
A) it produces light.
B) a solid appears when two solutions are mixed.
C) bubbles start to form when two substances are mixed.
D) all of these

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is one of the classes of pure substances?
A) compound
B) homogeneous mixture
C) solution
D) heterogeneous mixture
A) compound
B) homogeneous mixture
C) solution
D) heterogeneous mixture

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
13
In the balanced equation, 2 Al + 6 HCl 2 AlCl3 + 3 H2, the sum of the coefficients of the reactants is
A)5
B)8
C)13
D)none of these
A)5
B)8
C)13
D)none of these

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
14
The element whose name is derived from the Latin aurum, meaning shining dawn
A) gold.
B) aluminum.
C) silver.
D) chromium.
A) gold.
B) aluminum.
C) silver.
D) chromium.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
15
Separating a mixture of iron and sulfur can be done
A) by filtration.
B) dissolving in water.
C) with a magnet.
D) by burning.
A) by filtration.
B) dissolving in water.
C) with a magnet.
D) by burning.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
16
Which is a chemical property?
A) boiling point
B) state
C) odor
D) flammability
A) boiling point
B) state
C) odor
D) flammability

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following is not a chemical change?
A) burning charcoal
B) rusting iron
C) melting ice
D) baking bread
A) burning charcoal
B) rusting iron
C) melting ice
D) baking bread

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
18
The equation, 2 C(s) + O2(g) 2 CO(g), tells us
A) the number of atoms of each kind in reactants and products is the same.
B) carbon monoxide (CO) is a product.
C) two atoms of carbon undergo reaction.
D) all of these
A) the number of atoms of each kind in reactants and products is the same.
B) carbon monoxide (CO) is a product.
C) two atoms of carbon undergo reaction.
D) all of these

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
19
Which term describes energy?
A) motion
B) heat
C) light
D) all of these
A) motion
B) heat
C) light
D) all of these

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following elements is a metal?
A) Ca, calcium
B) Na, sodium
C) Hg, mercury
D) all of these
A) Ca, calcium
B) Na, sodium
C) Hg, mercury
D) all of these

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
21
How would you separate a mixture of salt, sand, and water?
A) by filtration, followed by evaporation
B) freezing, followed by melting
C) separating with tweezers, followed by evaporation
D) by filtration, followed by burning
A) by filtration, followed by evaporation
B) freezing, followed by melting
C) separating with tweezers, followed by evaporation
D) by filtration, followed by burning

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
22
What prefix is the largest?
A) mega
B) centi
C) micro
D) kilo
A) mega
B) centi
C) micro
D) kilo

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
23
Potential energy is defined as
A) heat energy.
B) energy associated with motion.
C) stored energy.
D) the ability to do work.
A) heat energy.
B) energy associated with motion.
C) stored energy.
D) the ability to do work.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
24
For which of the following is it necessary that there be a definite composition which cannot vary?
A) mixture
B) solution
C) compound
D) colloid
A) mixture
B) solution
C) compound
D) colloid

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
25
The quantity 10-9 (one billionth) is designated by the prefix
A) pico.
B) nano.
C) centi.
D) mega.
A) pico.
B) nano.
C) centi.
D) mega.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is not a pure substance?
A) pure gold
B) clean air
C) refined sugar
D) distilled water
A) pure gold
B) clean air
C) refined sugar
D) distilled water

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following would convert 15 L of gasoline to gallons? (1.06 qt = 1 L ; 4 qts = 1 gal)
A) (15) (1.06/1) (1/4)
B) (15) (1/1.06) (4/1)
C) (15) (1.06/1) (4/1)
D) (15) (1/1.06) (1/4)
A) (15) (1.06/1) (1/4)
B) (15) (1/1.06) (4/1)
C) (15) (1.06/1) (4/1)
D) (15) (1/1.06) (1/4)

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
28
Which state of matter is composed of charged particles which are dramatically affected by electric and magnetic fields?
A) solids
B) liquids
C) gases
D) plasmas
A) solids
B) liquids
C) gases
D) plasmas

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
29
How many chemical formulas are in this chemical equation? P4(s) + 6 F2(g) 4 PF3(g)
A) 2
B) 3
C) 4
D) 11
A) 2
B) 3
C) 4
D) 11

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
30
How does the known number of nonmetals compare to that of metals?
A) There are fewer metals.
B) There are an equal number of each.
C) There are fewer nonmetals.
D) This cannot be predicted because not all metals and nonmetals have been discovered.
A) There are fewer metals.
B) There are an equal number of each.
C) There are fewer nonmetals.
D) This cannot be predicted because not all metals and nonmetals have been discovered.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
31
A person weighs 165 lbs. Which of the following would calculate their mass in kilograms if 2.2 lbs = 1 kg?
A) 165 2.2
B) 165 ÷ 2.2
C) 2.2 ÷ 165
D) 165 + 2.2
A) 165 2.2
B) 165 ÷ 2.2
C) 2.2 ÷ 165
D) 165 + 2.2

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following is a compound?
A) mercury
B) blood
C) sugar
D) air
A) mercury
B) blood
C) sugar
D) air

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following is a physical property?
A) freezing point
B) color
C) odor
D) all of the above
A) freezing point
B) color
C) odor
D) all of the above

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following is a physical change?
A) souring of milk
B) ripening of fruit
C) frying an egg
D) melting
A) souring of milk
B) ripening of fruit
C) frying an egg
D) melting

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
35
A pure substance which can be decomposed into two or more pure substances is a(n)
A) element.
B) compound.
C) mixture.
D) colloid.
A) element.
B) compound.
C) mixture.
D) colloid.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following is an SI unit of ?
A) pound
B) kilogram
C) quart
D) calorie
A) pound
B) kilogram
C) quart
D) calorie

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
37
How many phosphorus atoms are in the formula H3PO4?
A) 4
B) 3
C) 7
D) 1
A) 4
B) 3
C) 7
D) 1

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
38
The simplest form of matter is a(n)
A) element.
B) mixture.
C) compound.
D) solution.
A) element.
B) mixture.
C) compound.
D) solution.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
39
How many categories of pure substances exist?
A) 2
B) 3
C) thousands
D) about 100
A) 2
B) 3
C) thousands
D) about 100

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
40
An example of a homogeneous mixture is
A) oil in water.
B) a salt water solution.
C) a suspension.
D) a pure substance.
A) oil in water.
B) a salt water solution.
C) a suspension.
D) a pure substance.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
41
The chemical symbol for copper is_________.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following has the highest kinetic energy?
A) boulder on the top of hill
B) water behind a dam
C) a ball falling from a 3 story building
D) a piece of wood
A) boulder on the top of hill
B) water behind a dam
C) a ball falling from a 3 story building
D) a piece of wood

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following is an example of a chemical change?
A) boiling water
B) iodine sublimating
C) barbecuing a steak
D) breaking a piece of glass
A) boiling water
B) iodine sublimating
C) barbecuing a steak
D) breaking a piece of glass

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
44
There are ____________mg in exactly 10g.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
45
Identify the nonmetal among those listed below.
A) Fe
B) Na
C) S
D) Ag
A) Fe
B) Na
C) S
D) Ag

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
46
Glucose has the chemical formula C6H12O6. In one molecule of glucose there are 24 atoms.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
47
Mg is the chemical symbol for ____________________.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
48
The SI multiple of 10-3 is indicated in a unit with the common prefix _________.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
49
The most common unit of volume used in chemistry is the millimeter.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
50
MATCHING
Use the pictures below to answer the following questions.
Which figure above depicts a heterogeneous mixture?
A)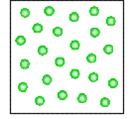
B)
C)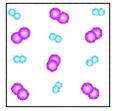
D)
Use the pictures below to answer the following questions.
Which figure above depicts a heterogeneous mixture?
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
51
What is the coefficient in front of iron when the following equation is balanced? Fe + O2 Fe2O3
A) 1
B) 2
C) 4
D) 6
A) 1
B) 2
C) 4
D) 6

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
52
A pure substance which can be decomposed into two or more pure substances is called a mixture.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
53
What kind of change is depicted in the following image? 

A) chemical change
B) physical change
C) both a chemical change and a physical change
D) There is no change shown in the image.


A) chemical change
B) physical change
C) both a chemical change and a physical change
D) There is no change shown in the image.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
54
MATCHING
Use the pictures below to answer the following questions.
Which figure above depicts an element?
A)
B)
C)
D)
Use the pictures below to answer the following questions.
Which figure above depicts an element?
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
55
MATCHING
Use the pictures below to answer the following questions.
Which figure above depicts a compound?
A)
B)
C)
D)
Use the pictures below to answer the following questions.
Which figure above depicts a compound?
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
56
How many millimeters are in 100 cm?
A) 10
B) 1000
C) 100
D) 1
A) 10
B) 1000
C) 100
D) 1

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
57
MATCHING
Use the pictures below to answer the following questions.
Which figure above depicts a homogeneous mixture?
A)
B)
C)
D)
Use the pictures below to answer the following questions.
Which figure above depicts a homogeneous mixture?
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
58
The density of copper is 8.96 g/mL and that of gold is 19.3 g/mL. The ratio of the mass of a 10 mL block of copper to a 10 mL block of gold is 0.464.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
59
In order to convert a measurement for the element mercury from mass to volume, one would multiply the starting measurement by the following factor. 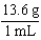
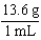

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck


