Deck 25: Amines
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/58
Play
Full screen (f)
Deck 25: Amines
1
Arrange the following compounds in order of decreasing boiling point, putting the compound with the highest boiling point first.
A) I > II > III
B) I > III > II
C) III > I > II
D) III > II > I
A) I > II > III
B) I > III > II
C) III > I > II
D) III > II > I
III > I > II
2
What is the IUPAC name of the following compound? 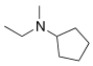
A) N-ethyl-N-methylcyclopentanamine
B) N-cyclopentyl-N-methylethanamine
C) N-methyl-N-ethylcyclopentylamine
D) N-ethyl-N-methylpentanamine

A) N-ethyl-N-methylcyclopentanamine
B) N-cyclopentyl-N-methylethanamine
C) N-methyl-N-ethylcyclopentylamine
D) N-ethyl-N-methylpentanamine
N-ethyl-N-methylcyclopentanamine
3
What is the common name of the following compound? 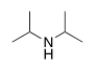
A) diisopropylamine
B) dipropylamine
C) diisopropanamine
D) dibutylamine

A) diisopropylamine
B) dipropylamine
C) diisopropanamine
D) dibutylamine
diisopropylamine
4
What is the IUPAC name of the following compound? 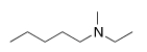
A) N-ethyl-N-methylpentylamine
B) N-ethyl-N-methyl-1-pentanamine
C) N-methyl-3-octanamine
D) N-ethyl-2-heptanamine

A) N-ethyl-N-methylpentylamine
B) N-ethyl-N-methyl-1-pentanamine
C) N-methyl-3-octanamine
D) N-ethyl-2-heptanamine

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following amines are classified as tertiary (3°) amines? 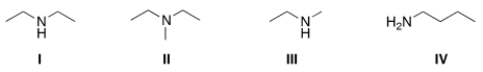
A) I
B) II
C) III
D) IV
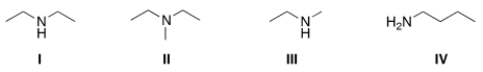
A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following amines are classified as secondary (2°) amines? 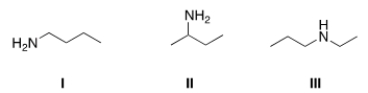
A) I
B) II
C) III
D) I and II

A) I
B) II
C) III
D) I and II

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
7
What is the correct assignment of the names of the following aromatic amines? 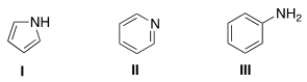
A) I = pyrrolidine; II = pyrimidine; III = aniline.
B) I = pyrrole; II = pyrimidine; III = anisole.
C) I = pyrrolidine; II = pyridine; III =aniline.
D) I = pyrrole; II = pyridine; III = aniline.

A) I = pyrrolidine; II = pyrimidine; III = aniline.
B) I = pyrrole; II = pyrimidine; III = anisole.
C) I = pyrrolidine; II = pyridine; III =aniline.
D) I = pyrrole; II = pyridine; III = aniline.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
8
What is the IUPAC name of the following compound? 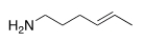
A) (Z)-4-hexen-1-amine
B) (E)-4-hexen-1-amine
C) (E)-2-hexen-6-amine
D) (Z)-2-hexen-6-amine

A) (Z)-4-hexen-1-amine
B) (E)-4-hexen-1-amine
C) (E)-2-hexen-6-amine
D) (Z)-2-hexen-6-amine

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
9
Although an amine nitrogen atom containing an electron pair and bonded to three different groups is technically a stereogenic center, the chirality of the amine nitrogen is often ignored. Why is that?
A) Because four bonds are needed to define a stereogenic center.
B) Because chirality only exists with the tetrahedral carbon atoms.
C) Because there is usually slow interconversion between the two isomeric forms at room temperature.
D) Because there is usually rapid interconversion between the two isomeric forms at room temperature.
A) Because four bonds are needed to define a stereogenic center.
B) Because chirality only exists with the tetrahedral carbon atoms.
C) Because there is usually slow interconversion between the two isomeric forms at room temperature.
D) Because there is usually rapid interconversion between the two isomeric forms at room temperature.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
10
What is the approximate bond angle of the substituents around a nitrogen atom in amines?
A) 90°
B) 109.5°
C) 120°
D) 180°
A) 90°
B) 109.5°
C) 120°
D) 180°

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
11
What is the IUPAC name of the following compound? 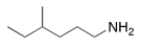
A) 3-methyl-1-hexanamine
B) 4-methyl-1-hexylamine
C) 4-methyl-1-hexanamine
D) 3-methyl-6-hexylamine

A) 3-methyl-1-hexanamine
B) 4-methyl-1-hexylamine
C) 4-methyl-1-hexanamine
D) 3-methyl-6-hexylamine

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
12
What is the IUPAC name of the following compound? 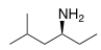
A) (S)-methyl-4-hexanamine
B) (S)-5-methyl-3-hexanamine
C) (R)-2-methyl-4-hexanamine
D) (R)-5-methyl-3-hexanamine

A) (S)-methyl-4-hexanamine
B) (S)-5-methyl-3-hexanamine
C) (R)-2-methyl-4-hexanamine
D) (R)-5-methyl-3-hexanamine

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
13
What is the IUPAC name of the following compound? 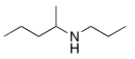
A) 1-methyl-N-propyl-1-propanamine
B) 4-methyl-4-heptanamine
C) 2-propyl-3-hexanamine
D) N-propyl-2-pentanamine

A) 1-methyl-N-propyl-1-propanamine
B) 4-methyl-4-heptanamine
C) 2-propyl-3-hexanamine
D) N-propyl-2-pentanamine

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
14
What is the common name of the following compound? 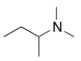
A) dimethylisobutylamine
B) butyldimethylamine
C) N,N-dimethylbutanamine
D) sec-butyldimethylamine

A) dimethylisobutylamine
B) butyldimethylamine
C) N,N-dimethylbutanamine
D) sec-butyldimethylamine

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
15
What is the IUPAC name of the following compound? 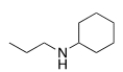
A) N-propylhexanamine
B) N-propylaniline
C) N-ethylcyclohexylamine
D) N-propylcyclohexanamine

A) N-propylhexanamine
B) N-propylaniline
C) N-ethylcyclohexylamine
D) N-propylcyclohexanamine

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
16
Why should the chirality of an ammonium salt with four different groups on the nitrogen atom not be ignored?
A) Because there is rapid interconversion between the two isomeric forms at room temperature.
B) Because interconversion cannot occur between the two isomeric forms at room temperature.
C) Because the compound would be a meso compound.
D) Because the compound would be a racemic mixture.
A) Because there is rapid interconversion between the two isomeric forms at room temperature.
B) Because interconversion cannot occur between the two isomeric forms at room temperature.
C) Because the compound would be a meso compound.
D) Because the compound would be a racemic mixture.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
17
What is the common name of the following compound? 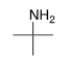
A) isopropylamine
B) 2-methyl-2-propanamine
C) tert-butylamine
D) isobutylamine

A) isopropylamine
B) 2-methyl-2-propanamine
C) tert-butylamine
D) isobutylamine

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
18
What is the common name of the following compound? 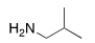
A) isopropylamine
B) sec-butylamine
C) 2-methyl-1-propanamine
D) isobutylamine

A) isopropylamine
B) sec-butylamine
C) 2-methyl-1-propanamine
D) isobutylamine

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following amines are classified as primary (1°) amines? 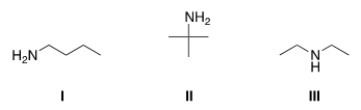
A) I
B) II
C) III
D) I and II

A) I
B) II
C) III
D) I and II

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
20
What is the common name of the following compound? 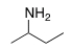
A) isopropylamine
B) sec-butylamine
C) isobutylamine
D) tert-butylamine

A) isopropylamine
B) sec-butylamine
C) isobutylamine
D) tert-butylamine

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
21
Why is direct nucleophilic substitution of an alkyl halide with NH3 not a very useful method for preparing primary amines?
A) NH3 is not a nucleophile.
B) Elimination will occur.
C) NH3 is too bulky to act as a nucleophile.
D) Polyalkylation of the amine will result in multiple products.
A) NH3 is not a nucleophile.
B) Elimination will occur.
C) NH3 is too bulky to act as a nucleophile.
D) Polyalkylation of the amine will result in multiple products.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
22
What is the major organic product obtained in the following reaction? 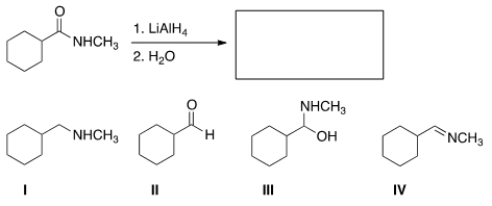
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
23
Predict the product(s) of the following reaction. 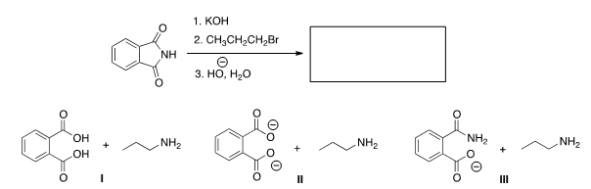
A) I
B) II
C) III
D) None of the choices

A) I
B) II
C) III
D) None of the choices

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
24
Why are alkylamines more basic than arylamines?
A) The lone pair electrons are localized in alkylamines and delocalized in arylamines.
B) The lone pair electrons are delocalized in alkylamines and localized in arylamines.
C) The lone pair electrons are less readily available in alkylamines.
D) The lone pair electrons are more readily available in arylamines.
A) The lone pair electrons are localized in alkylamines and delocalized in arylamines.
B) The lone pair electrons are delocalized in alkylamines and localized in arylamines.
C) The lone pair electrons are less readily available in alkylamines.
D) The lone pair electrons are more readily available in arylamines.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
25
The mass spectrum of an amine shows a parent peak with an odd mass for the molecular ion. What does this tell you about the amine?
A) The amine is a primary amine.
B) The amine is a secondary amine.
C) The amine contains an even number of N atoms.
D) The amine contains an odd number of N atoms.
A) The amine is a primary amine.
B) The amine is a secondary amine.
C) The amine contains an even number of N atoms.
D) The amine contains an odd number of N atoms.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
26
What is the major organic product obtained in the following reaction? 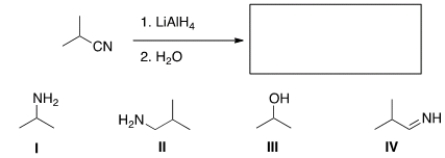
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
27
Rank the following compounds in order of increasing basicity, putting the least basic compound first. 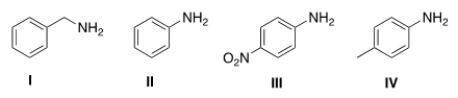
A) III < II < IV < I
B) II < III < IV < I
C) I < IV < II < III
D) III < I < II < IV

A) III < II < IV < I
B) II < III < IV < I
C) I < IV < II < III
D) III < I < II < IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
28
Arrange the following amines in order of decreasing water solubility, putting the most soluble amine first. 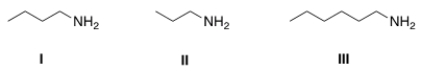
A) I > II > III
B) II > I > III
C) III > I > II
D) II > III > I

A) I > II > III
B) II > I > III
C) III > I > II
D) II > III > I

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
29
What is the major organic product obtained in the following reaction? 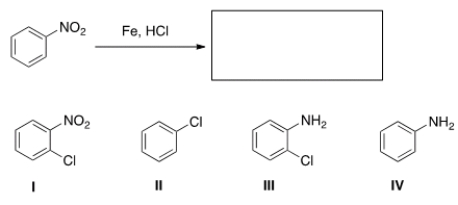
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
30
What is the major organic product obtained in the following reaction? 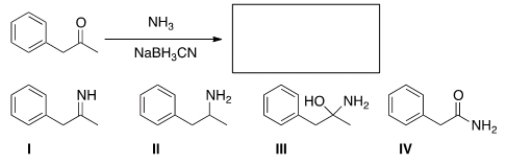
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
31
A compound with molecular formula C6H15N exhibits a singlet at 0.9 (1H), a triplet at 1.10 (3H), a singlet at 1.15 (9H), and a quartet at 2.6 (2H) in its 1HNMR spectrum. Its IR spectrum shows one medium absorption band near 3400 cm-1. What is the structure of this compound? 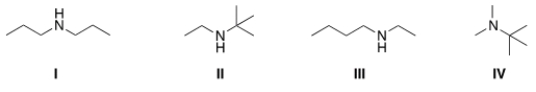
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
32
In the preparation of primary amines, how can direct nucleophilic substitution between NH3 and alkyl halide be made more practical than reacting NH3 and the alkyl halide in a 1:1 ratio?
A) Use a large excess of NH3.
B) Use a large excess of alkyl halide.
C) Use a limited amount of NH3.
D) Make the alkyl halide sterically hindered.
A) Use a large excess of NH3.
B) Use a large excess of alkyl halide.
C) Use a limited amount of NH3.
D) Make the alkyl halide sterically hindered.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
33
Rank the following compounds in order of increasing basicity, putting the least basic first.
A) II < III < IV < I
B) I < II < III < IV
C) IV < III < II < I
D) II < I < III < IV
A) II < III < IV < I
B) I < II < III < IV
C) IV < III < II < I
D) II < I < III < IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following alkyl halides cannot be used to prepare primary amines by the Gabriel synthesis?
A) 2-bromo-2-methylbutane
B) 1-bromo-2-methylbutane
C) 2-bromo-3-methylbutane
D) 1-bromo-3-methylbutane
A) 2-bromo-2-methylbutane
B) 1-bromo-2-methylbutane
C) 2-bromo-3-methylbutane
D) 1-bromo-3-methylbutane

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
35
Rank the following compounds in order of decreasing basicity, putting the most basic compound first. 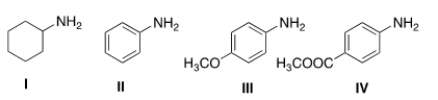
A) II > I > III > IV
B) I > II > III > IV
C) I > III > II > IV
D) IV > II > III > I

A) II > I > III > IV
B) I > II > III > IV
C) I > III > II > IV
D) IV > II > III > I

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
36
Arrange the following compounds in order of increasing boiling point, putting the compound with the least boiling point first. 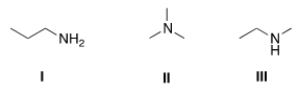
A) I < II < III
B) II < I < III
C) I < III < II
D) II < III < I

A) I < II < III
B) II < I < III
C) I < III < II
D) II < III < I

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
37
Why is the N-H bond of an imide especially acidic?
A) The conjugate base is stabilized by electron-donating inductive effect.
B) The conjugate base is stabilized by resonance.
C) The conjugate acid is stabilized by resonance.
D) The conjugate base is stabilized by intramolecular hydrogen bonding.
A) The conjugate base is stabilized by electron-donating inductive effect.
B) The conjugate base is stabilized by resonance.
C) The conjugate acid is stabilized by resonance.
D) The conjugate base is stabilized by intramolecular hydrogen bonding.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
38
Why are 1°, 2°, and 3° alkylamines more basic than ammonia (NH3)?
A) Because of the electron-withdrawing inductive effect of the alkyl groups.
B) Because of the steric hindrance of the alkyl groups.
C) Because of the resonance delocalization of the alkyl groups.
D) Because of electron-donating inductive effect of the alkyl groups.
A) Because of the electron-withdrawing inductive effect of the alkyl groups.
B) Because of the steric hindrance of the alkyl groups.
C) Because of the resonance delocalization of the alkyl groups.
D) Because of electron-donating inductive effect of the alkyl groups.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
39
What is a typical characteristic absorption in the IR spectrum of a primary amine?
A) Two N-H absorptions at 2500-2600 cm-1
B) One N-H absorption at 2500-2600 cm-1
C) Two N-H absorptions at 3300-3500 cm-1
D) One N-H absorption at 3300-3500 cm-1
A) Two N-H absorptions at 2500-2600 cm-1
B) One N-H absorption at 2500-2600 cm-1
C) Two N-H absorptions at 3300-3500 cm-1
D) One N-H absorption at 3300-3500 cm-1

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
40
What is the name given to naturally occurring amines derived from plant sources?
A) Enamines
B) Imines
C) Alkaloids
D) Alkamines
A) Enamines
B) Imines
C) Alkaloids
D) Alkamines

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
41
Predict the major product of the following reaction. 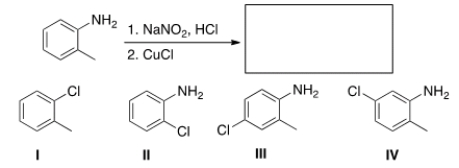
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
42
Predict the major organic product of the following reaction. 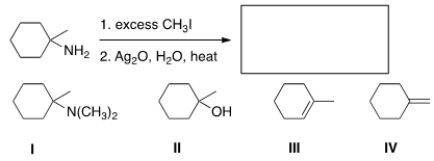
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
43
Select the reagent(s) required for the following transformation. 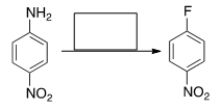
A) NaF
B) (1) NaNO2, HCl; (2) F2
C) (1) NaNO2, HCl; (2) HBF4
D) F2

A) NaF
B) (1) NaNO2, HCl; (2) F2
C) (1) NaNO2, HCl; (2) HBF4
D) F2

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
44
Why is piperidine a stronger base than pyridine?
A) The lone pair of electrons in pyridine is part of the delocalized system.
B) Aromatic compounds are always less basic than non-aromatic compounds.
C) The lone pair of electrons in piperidine is in an sp3 hybrid orbital; the lone pair of electrons in pyridine is in an sp hybrid orbital.
D) The lone pair of electrons in piperidine is in an sp3 hybrid orbital; the lone pair of electrons in pyridine is in an sp2 hybrid orbital.
A) The lone pair of electrons in pyridine is part of the delocalized system.
B) Aromatic compounds are always less basic than non-aromatic compounds.
C) The lone pair of electrons in piperidine is in an sp3 hybrid orbital; the lone pair of electrons in pyridine is in an sp hybrid orbital.
D) The lone pair of electrons in piperidine is in an sp3 hybrid orbital; the lone pair of electrons in pyridine is in an sp2 hybrid orbital.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
45
Rank the nitrogen atoms in chloroquine, shown below, in order of decreasing basicity, putting the most basic nitrogen atom first. 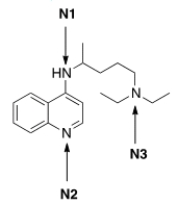
A) N1 > N2 > N3
B) N2 > N1 > N3
C) N3 > N2 > N1
D) N3 > N1 > N2

A) N1 > N2 > N3
B) N2 > N1 > N3
C) N3 > N2 > N1
D) N3 > N1 > N2

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
46
Predict the major organic product of the following reaction. 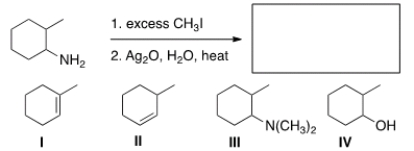
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
47
Predict the major product of the following reaction. 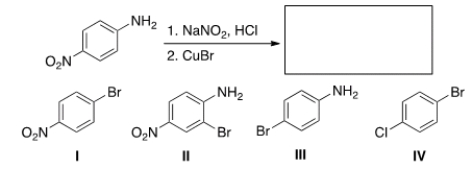
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
48
Why is pyrrole much less basic than pyridine?
A) The lone pair of electrons in pyrrole is located on an sp2 orbital.
B) The lone pair of electrons in pyrrole is part of the aromatic system.
C) The lone pair of electrons in pyrrole is not part of the aromatic system.
D) The pKa of the conjugate acid of pyrrole is much greater than the conjugate acid of pyridine.
A) The lone pair of electrons in pyrrole is located on an sp2 orbital.
B) The lone pair of electrons in pyrrole is part of the aromatic system.
C) The lone pair of electrons in pyrrole is not part of the aromatic system.
D) The pKa of the conjugate acid of pyrrole is much greater than the conjugate acid of pyridine.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
49
Rank the following compounds in order of decreasing basicity, putting the most basic first. 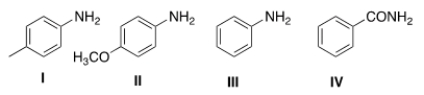
A) I > II > III > IV
B) I > III > II > IV
C) IV > III > I > II
D) II > I > III > IV

A) I > II > III > IV
B) I > III > II > IV
C) IV > III > I > II
D) II > I > III > IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
50
Select the reagent(s) required for the following transformation. 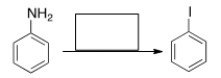
A) NaI
B) (1) NaNO2, HCl; (2) NaI
C) (1) NaNO2, HCl; (2) I2
D) I2

A) NaI
B) (1) NaNO2, HCl; (2) NaI
C) (1) NaNO2, HCl; (2) I2
D) I2

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
51
Rank the following compounds in increasing order of basicity, putting the least basic first. 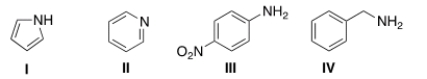
A) II < I < IV < III
B) I < III < II < IV
C) IV < II < III < I
D) I < II < III < IV

A) II < I < IV < III
B) I < III < II < IV
C) IV < II < III < I
D) I < II < III < IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
52
Histamine, a vasodilator, is responsible for a wide variety of physiological effects. Rank the three nitrogen atoms in histamine in increasing order of basicity, putting least basic nitrogen atom first. 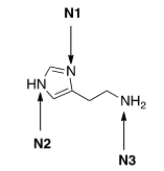
A) N2 < N1 < N3
B) N1 < N2 < N3
C) N3 < N1 < N2
D) N3 < N2 < N1

A) N2 < N1 < N3
B) N1 < N2 < N3
C) N3 < N1 < N2
D) N3 < N2 < N1

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
53
What starting materials are required to synthesize the following azo compound? 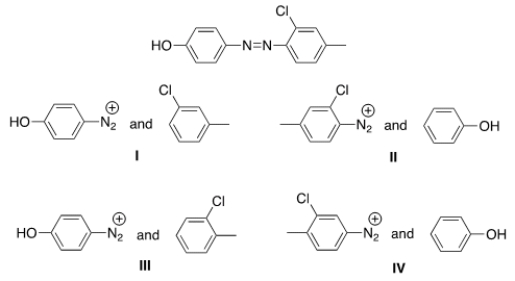
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
54
Predict the major organic product of the following reaction. 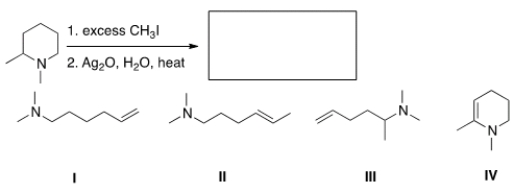
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
55
Select the reagent(s) required for the following transformation. 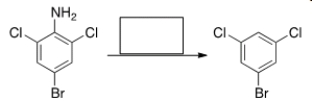
A) (1) NaNO2, HCl; (2) H2
B) (1) NaNO2, HCl; (2) H2O
C) (1) NaNO2, HCl; (2) H3PO4
D) (1) NaNO2, HCl; (2) H3PO2

A) (1) NaNO2, HCl; (2) H2
B) (1) NaNO2, HCl; (2) H2O
C) (1) NaNO2, HCl; (2) H3PO4
D) (1) NaNO2, HCl; (2) H3PO2

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
56
Predict the major product of the following reaction. 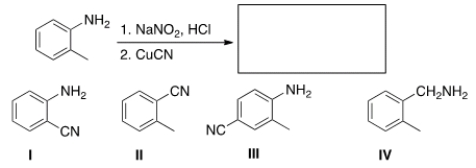
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
57
Predict the major product of the following reaction. 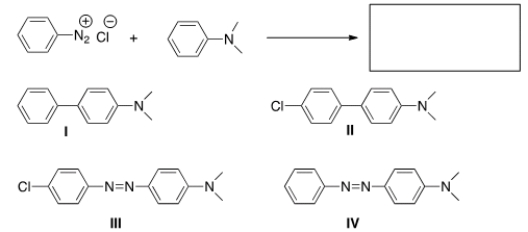
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
58
Predict the major organic product of the following reaction. 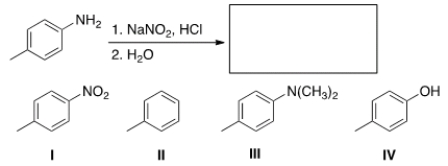
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck


