Deck 3: Protein Structure and Function
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/59
Play
Full screen (f)
Deck 3: Protein Structure and Function
1
The bonding of two amino acid molecules to form a larger molecule requires the ________.
A) release of a water molecule
B) release of a carbon dioxide molecule
C) addition of a carbon dioxide molecule
D) addition of a water molecule
E) addition of a water molecule and a carbon dioxide molecule
A) release of a water molecule
B) release of a carbon dioxide molecule
C) addition of a carbon dioxide molecule
D) addition of a water molecule
E) addition of a water molecule and a carbon dioxide molecule
A
2
Upon chemical analysis, a particular polypeptide was found to contain 100 amino acids. How many peptide bonds are present in this protein?
A) 101
B) 100
C) 99
D) 98
E) 97
A) 101
B) 100
C) 99
D) 98
E) 97
C
3
Refer to the accompanying figure to answer the following question(s). 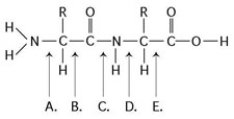
Which bond is closest to the amino group of the molecule?
A) A
B) B
C) C
D) D
E) E

Which bond is closest to the amino group of the molecule?
A) A
B) B
C) C
D) D
E) E
A
4
There are 20 different amino acids. What makes one amino acid different from another?
A) different side chains (R-groups) attached to a carboxyl carbon
B) different side chains (R-groups) attached to the amino groups
C) different side chains (R-groups) attached to an α carbon
D) different structural and optical isomers
E) different asymmetric carbons
A) different side chains (R-groups) attached to a carboxyl carbon
B) different side chains (R-groups) attached to the amino groups
C) different side chains (R-groups) attached to an α carbon
D) different structural and optical isomers
E) different asymmetric carbons

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
5
Which two functional groups are always found in amino acids?
A) ketone and methyl groups
B) carbonyl and amino groups
C) carboxyl and amino groups
D) amino and sulfhydryl groups
E) hydroxyl and carboxyl groups
A) ketone and methyl groups
B) carbonyl and amino groups
C) carboxyl and amino groups
D) amino and sulfhydryl groups
E) hydroxyl and carboxyl groups

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
6
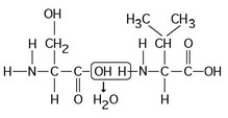 The chemical reaction illustrated in the accompanying figure ________.
The chemical reaction illustrated in the accompanying figure ________.A) is a hydrolysis reaction
B) results in a peptide bond
C) joins two fatty acids together
D) links two polymers to form a monomer
E) joins two phospholipids in a bilayer

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following involves an increase in entropy?
A) hydrolysis
B) reactions that join monomers
C) polymerization
D) chemical evolution
A) hydrolysis
B) reactions that join monomers
C) polymerization
D) chemical evolution

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
8
A peptide bond ________.
A) forms between the functional R-groups of different amino acids
B) forms between the central carbon and the amino R-group of a single amino acid
C) forms the primary structure of proteins
D) does not play a role in maintaining the tertiary structure of proteins
A) forms between the functional R-groups of different amino acids
B) forms between the central carbon and the amino R-group of a single amino acid
C) forms the primary structure of proteins
D) does not play a role in maintaining the tertiary structure of proteins

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
9
A hydrocarbon skeleton is covalently bonded to an amino group at one end and a carboxyl group at the other end. When placed in water this molecule would function ________.
A) only as an acid because of the carboxyl group
B) only as a base because of the amino group
C) as an acid and a base
D) as neither an acid nor a base
E) It is impossible to determine how it would function, based on the information provided.
A) only as an acid because of the carboxyl group
B) only as a base because of the amino group
C) as an acid and a base
D) as neither an acid nor a base
E) It is impossible to determine how it would function, based on the information provided.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
10
What component of amino acid structure varies among different amino acids?
A) the long carbon-hydrogen tails of the molecule
B) the presence of a central C atom
C) the components of the R-group
D) the glycerol molecule that forms the backbone of the amino acid
A) the long carbon-hydrogen tails of the molecule
B) the presence of a central C atom
C) the components of the R-group
D) the glycerol molecule that forms the backbone of the amino acid

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
11
Why are polymerization reactions endergonic? Polymerization reactions ________.
A) reduce entropy
B) release heat, making the reactant monomers move faster
C) release energy
D) are at equilibrium
A) reduce entropy
B) release heat, making the reactant monomers move faster
C) release energy
D) are at equilibrium

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
12
Refer to the accompanying figure to answer the following question(s). 
At which bond would water need to be added to achieve hydrolysis of the peptide, back to its component amino acids?
A) A
B) B
C) C
D) D
E) E

At which bond would water need to be added to achieve hydrolysis of the peptide, back to its component amino acids?
A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
13
Suppose you discovered a new amino acid. Its R-group contains only hydrogen and carbon atoms. Predict the behavior of this amino acid.
A) It is hydrophobic.
B) It is hydrophilic.
C) Relative to the amino acids found in organisms, its interactions with water will be intermediate.
D) Relative to the amino acids found in organisms, its interactions with water will be very high.
A) It is hydrophobic.
B) It is hydrophilic.
C) Relative to the amino acids found in organisms, its interactions with water will be intermediate.
D) Relative to the amino acids found in organisms, its interactions with water will be very high.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
14
Amino acids are acids because they always possess which functional group?
A) amino
B) carbonyl
C) carboxyl
D) phosphate
E) hydroxyl
A) amino
B) carbonyl
C) carboxyl
D) phosphate
E) hydroxyl

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
15
In solution, why do hydrolysis reactions occur more readily than condensation reactions?
A) Hydrolysis increases entropy and is exergonic.
B) Hydrolysis decreases entropy and is endergonic.
C) Hydrolysis decreases entropy and is exergonic.
D) Hydrolysis increases entropy and is endergonic.
A) Hydrolysis increases entropy and is exergonic.
B) Hydrolysis decreases entropy and is endergonic.
C) Hydrolysis decreases entropy and is exergonic.
D) Hydrolysis increases entropy and is endergonic.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
16
Side chains of amino acids ________.
A) are all nonpolar
B) are nonpolar if they contain N or S
C) are all polar
D) may be polar or nonpolar
A) are all nonpolar
B) are nonpolar if they contain N or S
C) are all polar
D) may be polar or nonpolar

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
17
Refer to the accompanying figure to answer the following question(s). 
Which bond is a peptide bond?
A) A
B) B
C) C
D) D
E) E

Which bond is a peptide bond?
A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
18
At about pH 7 in most cells, what happens to the carboxyl R-group on an amino acid?
A) It acts as a base and gains a proton, giving it a positive charge.
B) It acts as an acid and loses a proton, giving it a negative charge.
C) It is oxidized and tends to act as an electron acceptor in redox reactions.
D) It remains neutral, like water, and does not have a charge.
A) It acts as a base and gains a proton, giving it a positive charge.
B) It acts as an acid and loses a proton, giving it a negative charge.
C) It is oxidized and tends to act as an electron acceptor in redox reactions.
D) It remains neutral, like water, and does not have a charge.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
19
At about pH 7 in most cells, what happens to the amino R-group on an amino acid?
A) It acts as a base and gains a proton, giving it a positive charge.
B) It acts as an acid and loses a proton, giving it a negative charge.
C) It is reduced and tends to act as an electron donor in redox reactions.
D) It remains neutral, like water, and does not have a charge.
A) It acts as a base and gains a proton, giving it a positive charge.
B) It acts as an acid and loses a proton, giving it a negative charge.
C) It is reduced and tends to act as an electron donor in redox reactions.
D) It remains neutral, like water, and does not have a charge.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
20
Which one of the following is NOT a component of each monomer used to make proteins?
A) a phosphorus atom, P
B) an amino functional group, NH2
C) a side chain, R
D) a carboxyl group, COOH
A) a phosphorus atom, P
B) an amino functional group, NH2
C) a side chain, R
D) a carboxyl group, COOH

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
21
Refer to the following paragraph and accompanying figure to answer the following question(s). 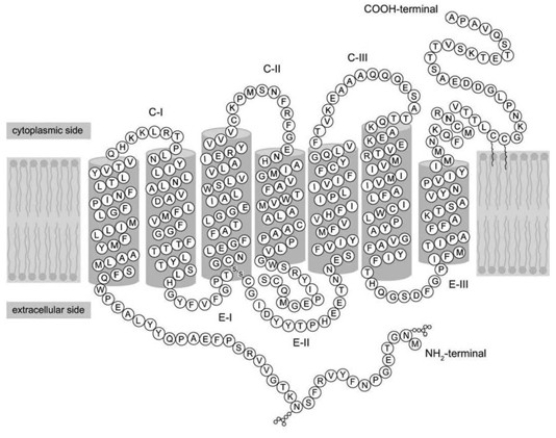
If you were reading off the sequence of amino acids in the figure to a biologist friend, what should the first three letters be?
A) M-N-G
B) A-P-A
C) It does not matter, since the protein has no polarity or directionality.

If you were reading off the sequence of amino acids in the figure to a biologist friend, what should the first three letters be?
A) M-N-G
B) A-P-A
C) It does not matter, since the protein has no polarity or directionality.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
22
The tertiary structure of a protein is the ________.
A) bonding together of several polypeptide chains by weak bonds
B) order in which amino acids are joined in a polypeptide chain
C) unique three-dimensional shape of the fully folded polypeptide
D) organization of a polypeptide chain into an α-helix or β-pleated sheet
E) overall protein structure resulting from the aggregation of two or more polypeptide subunits
A) bonding together of several polypeptide chains by weak bonds
B) order in which amino acids are joined in a polypeptide chain
C) unique three-dimensional shape of the fully folded polypeptide
D) organization of a polypeptide chain into an α-helix or β-pleated sheet
E) overall protein structure resulting from the aggregation of two or more polypeptide subunits

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
23
What type of interaction is directly responsible for the formation of secondary structure?
A) peptide bonds between adjacent amino acids
B) peptide bonds between nonadjacent amino acids
C) hydrogen bonds between sections of the polypeptide backbone
D) hydrogen bonds between side chains of amino acids
A) peptide bonds between adjacent amino acids
B) peptide bonds between nonadjacent amino acids
C) hydrogen bonds between sections of the polypeptide backbone
D) hydrogen bonds between side chains of amino acids

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
24
Aquaporins are proteins that control the passage of water molecules across a cell membrane. The protein forms a pore, or opening, in the membrane. You isolate what you think are two different molecules of aquaporin and determine that one of the proteins has a larger pore diameter than the second. Which of the following do you conclude?
A) These two forms of aquaporin will have identical sequences of amino acids.
B) These two forms of aquaporin will have different sequences of amino acids.
C) You will have to sequence the proteins to compare their primary structure, because it should have no effect on pore diameter.
D) These two forms of aquaporin have identical primary structure but differ in their tertiary structure.
A) These two forms of aquaporin will have identical sequences of amino acids.
B) These two forms of aquaporin will have different sequences of amino acids.
C) You will have to sequence the proteins to compare their primary structure, because it should have no effect on pore diameter.
D) These two forms of aquaporin have identical primary structure but differ in their tertiary structure.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
25
The following question(s) are based on the 15 molecules illustrated in the accompanying figure. Each molecule may be used once, more than once, or not at all. 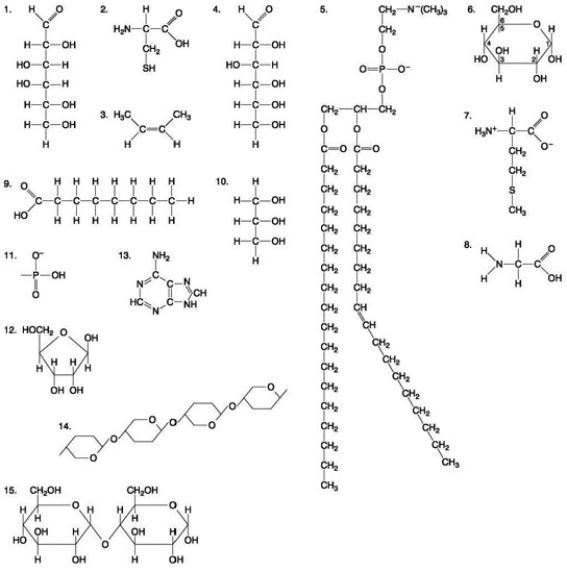
Which of the following molecules is an amino acid with a hydrophobic R-group or side chain?
A) 3
B) 7
C) 10
D) 12
E) 13

Which of the following molecules is an amino acid with a hydrophobic R-group or side chain?
A) 3
B) 7
C) 10
D) 12
E) 13

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
26
You are studying a protein that is shaped like a doughnut. The shape is a function of which level(s) of protein structure?
A) primary only
B) secondary only
C) tertiary only
D) secondary and tertiary only
E) primary, secondary, and tertiary
A) primary only
B) secondary only
C) tertiary only
D) secondary and tertiary only
E) primary, secondary, and tertiary

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
27
You have just sequenced a new protein found in mice and observe that sulfur-containing cysteine residues occur at regular intervals. What is the significance of this finding?
A) Cysteine residues are required for the formation of α-helices and β-pleated sheets.
B) It will be important to include cysteine in the diet of the mice.
C) Cysteine residues are involved in disulfide bridges that help form tertiary structure.
D) Cysteine causes bends, or angles, to occur in the tertiary structure of proteins.
A) Cysteine residues are required for the formation of α-helices and β-pleated sheets.
B) It will be important to include cysteine in the diet of the mice.
C) Cysteine residues are involved in disulfide bridges that help form tertiary structure.
D) Cysteine causes bends, or angles, to occur in the tertiary structure of proteins.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
28
The following question(s) are based on the 15 molecules illustrated in the accompanying figure. Each molecule may be used once, more than once, or not at all. 
Which type of interaction stabilizes the α-helix and the β-pleated sheet structures of proteins?
A) hydrophobic interactions
B) disulfide bonds
C) ionic bonds
D) hydrogen bonds
E) peptide bonds

Which type of interaction stabilizes the α-helix and the β-pleated sheet structures of proteins?
A) hydrophobic interactions
B) disulfide bonds
C) ionic bonds
D) hydrogen bonds
E) peptide bonds

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
29
When polymerization of a protein is complete, but a protein is still completely linear, what is the highest level of structure in the protein?
A) primary
B) secondary
C) tertiary
D) quaternary
A) primary
B) secondary
C) tertiary
D) quaternary

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
30
What is the location of the C-terminus of the protein in the figure?
A) extracellular
B) cytoplasm
C) embedded within the membrane
D) nucleus
A) extracellular
B) cytoplasm
C) embedded within the membrane
D) nucleus

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
31
The following question(s) are based on the 15 molecules illustrated in the accompanying figure. Each molecule may be used once, more than once, or not at all. 
Which of the following pairs of molecules could be joined together by a peptide bond in a dehydration reaction?
A) 2 and 3
B) 3 and 7
C) 7 and 8
D) 8 and 9
E) 12 and 13

Which of the following pairs of molecules could be joined together by a peptide bond in a dehydration reaction?
A) 2 and 3
B) 3 and 7
C) 7 and 8
D) 8 and 9
E) 12 and 13

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
32
The amino acids of the protein keratin are arranged predominantly in an α-helix. This secondary structure is stabilized by ________.
A) covalent bonds
B) peptide bonds
C) ionic bonds
D) polar bonds
E) hydrogen bonds
A) covalent bonds
B) peptide bonds
C) ionic bonds
D) polar bonds
E) hydrogen bonds

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
33
You disrupt all hydrogen bonds in a protein. What level of structure will be preserved?
A) primary structure
B) secondary structure
C) tertiary structure
D) quaternary structure
A) primary structure
B) secondary structure
C) tertiary structure
D) quaternary structure

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
34
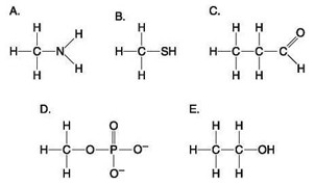 Which molecule shown above contains an amino functional group but is NOT an amino acid?
Which molecule shown above contains an amino functional group but is NOT an amino acid?A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
35
You determine the amino acid sequence of a protein and find it contains a long sequence of methionine, followed by a long sequence of proline, followed by a long sequence of valine. Using these data you predict the sequence of this protein's secondary structure will be ________.
A) beta sheets, then a region of no secondary structure, then beta sheets
B) alpha-helices, then a region of no secondary structure, then alpha helices
C) beta sheets, then a region of no secondary structure, then alpha-helices
D) alpha-helices, then a region of no secondary structure, then beta sheets
A) beta sheets, then a region of no secondary structure, then beta sheets
B) alpha-helices, then a region of no secondary structure, then alpha helices
C) beta sheets, then a region of no secondary structure, then alpha-helices
D) alpha-helices, then a region of no secondary structure, then beta sheets

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
36
The following question(s) are based on the 15 molecules illustrated in the accompanying figure. Each molecule may be used once, more than once, or not at all. 
Which bonds are created during the formation of the primary structure of a protein?
A) peptide bonds
B) hydrogen bonds
C) disulfide bonds
D) phosphodiester bonds
E) peptide bonds, hydrogen bonds, and disulfide bonds

Which bonds are created during the formation of the primary structure of a protein?
A) peptide bonds
B) hydrogen bonds
C) disulfide bonds
D) phosphodiester bonds
E) peptide bonds, hydrogen bonds, and disulfide bonds

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
37
How does primary protein structure affect the function of protein enzymes?
A) Substrates interact with R-groups at the enzyme's active site.
B) Substrates interact with R-groups at the enzyme's external surface.
C) Substrates interact with hydrophobic R-groups at any region of the enzyme.
D) Substrates permanently bind to R-groups at the enzyme's active site.
A) Substrates interact with R-groups at the enzyme's active site.
B) Substrates interact with R-groups at the enzyme's external surface.
C) Substrates interact with hydrophobic R-groups at any region of the enzyme.
D) Substrates permanently bind to R-groups at the enzyme's active site.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
38
Identify the location of the disulfide bond in the figure, located at the bottom of the third transmembrane segment. What is the name of the amino acids that are forming this bond?
A) cytosine
B) aspartic acid
C) cysteine
D) glycine
A) cytosine
B) aspartic acid
C) cysteine
D) glycine

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
39
Refer to the figure. Which level of structure is maintained by the disulfide bond?
A) primary
B) secondary
C) tertiary
D) quaternary
A) primary
B) secondary
C) tertiary
D) quaternary

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
40
A series of hydrophobic side chains will congregate together as a protein folds in an aqueous solution and be stabilized by ________.
A) disulfide bonds
B) van der Waals interactions
C) hydrogen bonds
D) quaternary structure bonds
A) disulfide bonds
B) van der Waals interactions
C) hydrogen bonds
D) quaternary structure bonds

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
41
Changing a single amino acid in a protein consisting of 325 amino acids would ________.
A) alter the primary structure of the protein but not its tertiary structure or function
B) cause the tertiary structure of the protein to unfold
C) always alter the biological activity or function of the protein
D) always alter the secondary structure of the protein and disrupt its biological activity
E) always alter the primary structure of the protein, sometimes alter the tertiary structure of the protein, and sometimes affect its biological activity
A) alter the primary structure of the protein but not its tertiary structure or function
B) cause the tertiary structure of the protein to unfold
C) always alter the biological activity or function of the protein
D) always alter the secondary structure of the protein and disrupt its biological activity
E) always alter the primary structure of the protein, sometimes alter the tertiary structure of the protein, and sometimes affect its biological activity

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
42
At which level of protein structure are interactions between the side chains (R-groups) most important?
A) primary
B) secondary
C) tertiary
D) quaternary
E) primary, secondary, tertiary, and quaternary
A) primary
B) secondary
C) tertiary
D) quaternary
E) primary, secondary, tertiary, and quaternary

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
43
Van der Waals interactions may result when ________.
A) hybrid orbitals overlap
B) electrons are not symmetrically distributed in a molecule
C) molecules held by ionic bonds react with water
D) two polar covalent bonds react
E) a hydrogen atom loses an electron
A) hybrid orbitals overlap
B) electrons are not symmetrically distributed in a molecule
C) molecules held by ionic bonds react with water
D) two polar covalent bonds react
E) a hydrogen atom loses an electron

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
44
Proteins in biological systems ________.
A) store genetic information
B) link with other proteins to form bilayers in cell membranes
C) form high-energy intermediates such as ATP
D) catalyze reactions
A) store genetic information
B) link with other proteins to form bilayers in cell membranes
C) form high-energy intermediates such as ATP
D) catalyze reactions

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
45
What are prions?
A) mobile segments of DNA
B) tiny circular molecules of RNA that can infect plants
C) viral DNA that attaches itself to the host genome and causes disease
D) misfolded versions of normal protein that can cause disease
E) viruses that invade bacteria
A) mobile segments of DNA
B) tiny circular molecules of RNA that can infect plants
C) viral DNA that attaches itself to the host genome and causes disease
D) misfolded versions of normal protein that can cause disease
E) viruses that invade bacteria

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
46
The structural level of a protein LEAST affected by a disruption in hydrogen bonding is the ________.
A) primary level
B) secondary level
C) tertiary level
D) quaternary level
E) All structural levels are equally affected by a disruption in hydrogen bonding.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
47
An enzyme has a total of four active sites. When you denature the molecule and study its composition, you find that each active site occurs on a different polypeptide. Which of the following hypotheses does this observation support?
A) The enzyme is subject to regulation.
B) The enzyme requires a cofactor to function normally.
C) The protein's structure is affected by temperature and pH.
D) The protein has quaternary structure.
A) The enzyme is subject to regulation.
B) The enzyme requires a cofactor to function normally.
C) The protein's structure is affected by temperature and pH.
D) The protein has quaternary structure.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following is the strongest evidence that protein structure and function are correlated?
A) Proteins function best at certain temperatures.
B) Proteins have four distinct levels of structure and many functions.
C) Enzymes tend to be globular in shape.
D) Denatured (unfolded) proteins do not function normally.
A) Proteins function best at certain temperatures.
B) Proteins have four distinct levels of structure and many functions.
C) Enzymes tend to be globular in shape.
D) Denatured (unfolded) proteins do not function normally.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
49
The R-group, or side chain, of the amino acid serine is -CH2-OH. The R-group, or side chain, of the amino acid leucine is -CH2-CH-(CH3)2. Where would you expect to find these amino acids in a globular protein in aqueous solution?
A) Serine would be in the interior, and leucine would be on the exterior of the globular protein.
B) Leucine would be in the interior, and serine would be on the exterior of the globular protein.
C) Serine and leucine would both be in the interior of the globular protein.
D) Serine and leucine would both be on the exterior of the globular protein.
A) Serine would be in the interior, and leucine would be on the exterior of the globular protein.
B) Leucine would be in the interior, and serine would be on the exterior of the globular protein.
C) Serine and leucine would both be in the interior of the globular protein.
D) Serine and leucine would both be on the exterior of the globular protein.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
50
Normal hemoglobin is a tetramer, consisting of two molecules of β hemoglobin and two molecules of α hemoglobin. In sickle-cell disease, as a result of a single amino acid change, the mutant hemoglobin tetramers associate with each other and assemble into large fibers. Based on this information alone, we can conclude that sickle-cell hemoglobin exhibits ________.
A) only altered primary structure
B) only altered secondary structure
C) only altered tertiary structure
D) only altered quaternary structure
E) altered primary structure and altered quaternary structure; the secondary and tertiary structures may or may not be altered
A) only altered primary structure
B) only altered secondary structure
C) only altered tertiary structure
D) only altered quaternary structure
E) altered primary structure and altered quaternary structure; the secondary and tertiary structures may or may not be altered

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
51

The structure depicted the accompanying figure shows the ________.
A) 1-4 linkage of the α-glucose monomers of starch
B) 1-4 linkage of the β-glucose monomers of cellulose
C) double-helical structure of a DNA molecule
D) α-helix secondary structure of a polypeptide
E) β-pleated sheet secondary structure of a polypeptide

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
52
Refer to the following paragraph and accompanying figure to answer the following question(s). 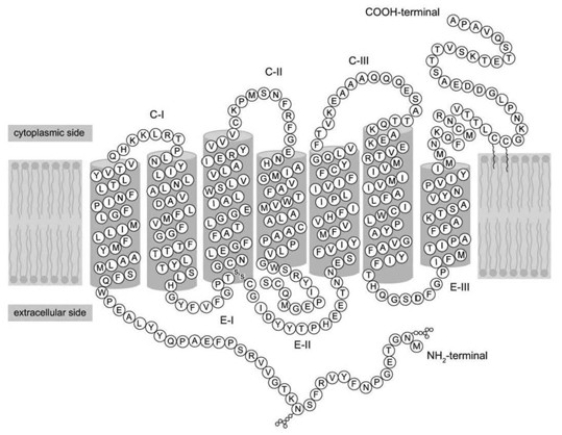
How many times does the protein in the figure cross the cell membrane?
A) 1
B) 3
C) 4
D) 7

How many times does the protein in the figure cross the cell membrane?
A) 1
B) 3
C) 4
D) 7

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
53
Which level of protein structure do the α-helix and the β-pleated sheet represent?
A) primary
B) secondary
C) tertiary
D) quaternary
E) primary, secondary, tertiary, and quaternary
A) primary
B) secondary
C) tertiary
D) quaternary
E) primary, secondary, tertiary, and quaternary

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
54
If cells are grown in a medium containing radioactive 35S, which of these molecules will be labeled?
A) phospholipids
B) nucleic acids
C) proteins
D) amylose
E) proteins and nucleic acids
A) phospholipids
B) nucleic acids
C) proteins
D) amylose
E) proteins and nucleic acids

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
55
What type of covalent bond between amino acid side chains (R-groups) functions in maintaining a polypeptide's specific three-dimensional shape?
A) ionic bond
B) hydrophobic interaction
C) van der Waals interaction
D) disulfide bond
E) hydrogen bond
A) ionic bond
B) hydrophobic interaction
C) van der Waals interaction
D) disulfide bond
E) hydrogen bond

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
56
The following questions are based on the 15 molecules illustrated in the accompanying figure. Each molecule may be used once, more than once, or not at all. 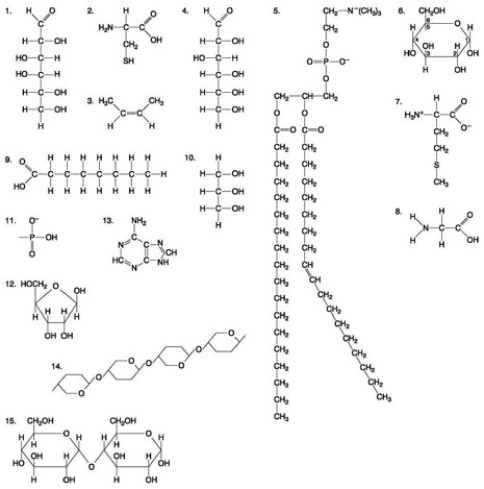 Which of the following molecules act as building blocks (monomers) of polypeptides?
Which of the following molecules act as building blocks (monomers) of polypeptides?
A) 1, 4, and 6
B) 2, 7, and 8
C) 7, 8, and 13
D) 11, 12, and 13
E) 12, 13, and 15
 Which of the following molecules act as building blocks (monomers) of polypeptides?
Which of the following molecules act as building blocks (monomers) of polypeptides?A) 1, 4, and 6
B) 2, 7, and 8
C) 7, 8, and 13
D) 11, 12, and 13
E) 12, 13, and 15

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
57
In a normal cellular protein, where would you expect to find a hydrophobic amino acid like valine?
A) in the interior of the folded protein, away from water
B) on the exterior surface of the protein, interacting with water
C) in the transmembrane portion interacting with lipid fatty-acid chains
D) in the interior of the folded protein, away from water, or in a transmembrane portion interacting with lipid fatty-acid chains
E) anywhere in the protein, with equal probability
A) in the interior of the folded protein, away from water
B) on the exterior surface of the protein, interacting with water
C) in the transmembrane portion interacting with lipid fatty-acid chains
D) in the interior of the folded protein, away from water, or in a transmembrane portion interacting with lipid fatty-acid chains
E) anywhere in the protein, with equal probability

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
58
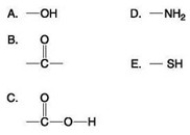 Which of the groups shown above is a functional group that helps stabilize proteins by forming covalent cross-links within or between protein molecules?
Which of the groups shown above is a functional group that helps stabilize proteins by forming covalent cross-links within or between protein molecules?A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
59
Misfolding of polypeptides is a serious problem in cells. Which of the following diseases are associated with an accumulation of misfolded polypeptides?
A) Alzheimer's only
B) Parkinson's only
C) diabetes mellitus only
D) Alzheimer's and Parkinson's only
E) Alzheimer's, Parkinson's, and diabetes mellitus
A) Alzheimer's only
B) Parkinson's only
C) diabetes mellitus only
D) Alzheimer's and Parkinson's only
E) Alzheimer's, Parkinson's, and diabetes mellitus

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck


