Deck 15: Carboxylic Acids and Esters
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/86
Play
Full screen (f)
Deck 15: Carboxylic Acids and Esters
1
What is the correct IUPAC name for the following compound? 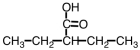
A)3-ethylbutanoic acid
B)2-ethylbutanoic acid
C)2-pentanoic acid
D)2-ethylpentanoic acid

A)3-ethylbutanoic acid
B)2-ethylbutanoic acid
C)2-pentanoic acid
D)2-ethylpentanoic acid
2-ethylbutanoic acid
2
What is the correct IUPAC name for the compound given below? 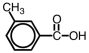
A)1-methyl-3-benzoic acid
B)3-methyl-1-benzoic acid
C)3-methylbenzoic acid
D)3-methylbenzoate

A)1-methyl-3-benzoic acid
B)3-methyl-1-benzoic acid
C)3-methylbenzoic acid
D)3-methylbenzoate
3-methylbenzoic acid
3
What reagent is necessary to complete the following reaction? 
A)Na
B)NaCl
C)NaOH
D)Na2O2

A)Na
B)NaCl
C)NaOH
D)Na2O2
NaOH
4
What is the IUPAC name for 5-bromobenzoic acid?
A)5-bromobenzoate
B)1-bromo-5-benzoic acid
C)2-bromobenzoic acid
D)3-bromobenzoic acid
A)5-bromobenzoate
B)1-bromo-5-benzoic acid
C)2-bromobenzoic acid
D)3-bromobenzoic acid

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
5
Two molecules of a carboxylic acid are joined together by two hydrogen bonds.This is referred to as a(n)____.
A)dimer
B)amide
C)ester
D)carboxylate
A)dimer
B)amide
C)ester
D)carboxylate

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
6
As CH3COOH dissolves in water,H3O+ and ____ are formed.
A)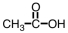
B)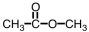
C)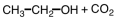
D)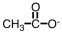
A)
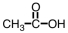
B)
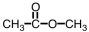
C)
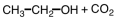
D)
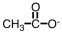

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following would you expect to have the highest boiling point?
A)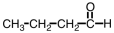
B)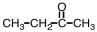
C)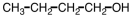
D)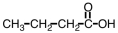
A)
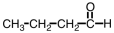
B)
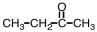
C)
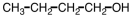
D)
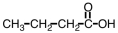

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
8
Which physical relationship is attributed to carboxylic acids?
A)Since the carboxylic acids evaporate quickly they all have a scent.
B)The boiling point of carboxylic acids increases with the molecular weight.
C)The carboxylic acids are wax-like solids in the solid state.
D)None of these responses are correct.
A)Since the carboxylic acids evaporate quickly they all have a scent.
B)The boiling point of carboxylic acids increases with the molecular weight.
C)The carboxylic acids are wax-like solids in the solid state.
D)None of these responses are correct.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following conditions would favor the carboxylate ion instead of the carboxylic acid?
A)low pH
B)high pH
C)both low pH and high pH
D)neither low pH or high pH
A)low pH
B)high pH
C)both low pH and high pH
D)neither low pH or high pH

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
10
The acids were among the first organic compounds studied.Why?
A)simplicity
B)color
C)aroma
D)abundance
A)simplicity
B)color
C)aroma
D)abundance

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is produced in the reaction shown below. 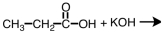
A)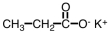
B)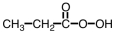
C)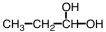
D)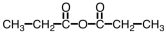
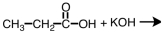
A)
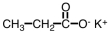
B)
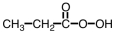
C)

D)
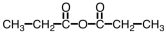

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
12
What is the name of the potassium salt of m-ethylbenzoic acid?
A)potassium m-ethylbenzoic acid
B)potassium m-ethylbenzoic
C)potassium m-ethylbenzoate
D)m-ethyl potassium benzoate
A)potassium m-ethylbenzoic acid
B)potassium m-ethylbenzoic
C)potassium m-ethylbenzoate
D)m-ethyl potassium benzoate

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
13
What is the IUPAC name of the compound shown below? 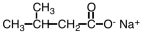
A)sodium 3-methylbutanonate
B)sodium 2-methylbutanonate
C)2-methyl sodium butanonate
D)3-methyl sodium butanonate
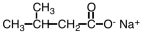
A)sodium 3-methylbutanonate
B)sodium 2-methylbutanonate
C)2-methyl sodium butanonate
D)3-methyl sodium butanonate

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
14
What is a source of oxalic acid?
A)rust stains
B)citrus fruits
C)spinach
D)pickles
A)rust stains
B)citrus fruits
C)spinach
D)pickles

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following is used to represent an organic acid?
A)COH
B)COC
C)COOH
D)CHO
A)COH
B)COC
C)COOH
D)CHO

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is used as a mold inhibitor in bakery goods?
A)calcium propionate
B)calcium citrate
C)sodium stearate
D)sodium formate
A)calcium propionate
B)calcium citrate
C)sodium stearate
D)sodium formate

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following compounds would be most soluble in water?
A)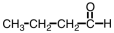
B)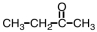
C)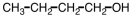
D)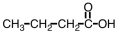
A)
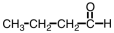
B)
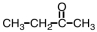
C)
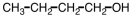
D)
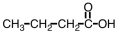

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is the structure for 2-bromopropanoic acid?
A)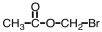
B)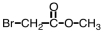
C)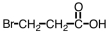
D)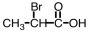
A)
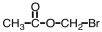
B)
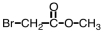
C)
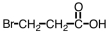
D)
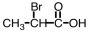

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
19
Which acid is responsible for the stinging sensation from the bite of a fire ant?
A)acetic acid
B)butyric acid
C)caproic acid
D)formic acid
A)acetic acid
B)butyric acid
C)caproic acid
D)formic acid

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is an ingredient in soaps?
A)sodium benzoate
B)sodium propionate
C)sodium stearate
D)zinc 10-undecylenate
A)sodium benzoate
B)sodium propionate
C)sodium stearate
D)zinc 10-undecylenate

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
21
What is the common name of the compound shown below? 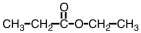
A)methyl propionate
B)propyl acetate
C)ethyl acetate
D)ethyl propionate
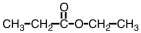
A)methyl propionate
B)propyl acetate
C)ethyl acetate
D)ethyl propionate

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
22
The IUPAC name of the ester formed from butanoic acid and ethanol is
A)butyl ethanate.
B)butyl ethanonate.
C)ethyl proponoate.
D)ethyl butanoate.
A)butyl ethanate.
B)butyl ethanonate.
C)ethyl proponoate.
D)ethyl butanoate.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following is the structure of methyl acetate?
A)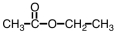
B)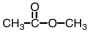
C)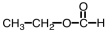
D)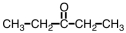
A)
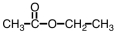
B)
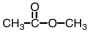
C)
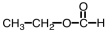
D)
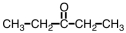

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
24
What would be the products if ethyl stearate were to undergo hydrolysis?
A)There would be no products formed.
B)The products would be ethylaldehyde and stearone.
C)The products would be ethanol and stearic acid.
D)The products would be ethyl stearate hydrate.
A)There would be no products formed.
B)The products would be ethylaldehyde and stearone.
C)The products would be ethanol and stearic acid.
D)The products would be ethyl stearate hydrate.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
25
Which ester would give CH3COOH and CH3CH2CH2CH2OH upon hydrolysis?
A)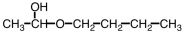
B)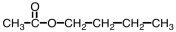
C)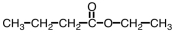
D)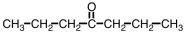
A)
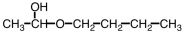
B)
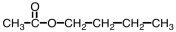
C)
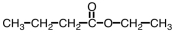
D)
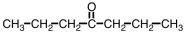

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
26
Give the IUPAC name for the compound shown below. 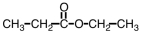
A)propyl ethanoate
B)propyl ethanate
C)ethyl acetate
D)ethyl propanoate
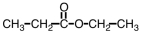
A)propyl ethanoate
B)propyl ethanate
C)ethyl acetate
D)ethyl propanoate

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
27
What are products of the following reaction? 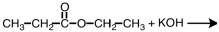
A)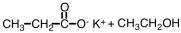
B)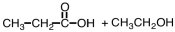
C)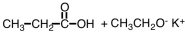
D)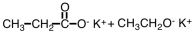
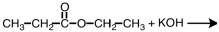
A)
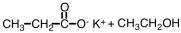
B)
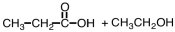
C)
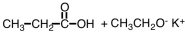
D)
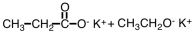

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
28
Nitrate esters are found in some
A)flavoring agents.
B)detergents.
C)food preservatives.
D)explosives.
A)flavoring agents.
B)detergents.
C)food preservatives.
D)explosives.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
29
What is the product of the following reaction? 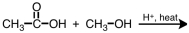
A)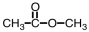
B)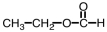
C)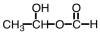
D)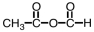
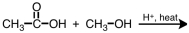
A)
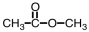
B)
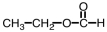
C)
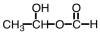
D)
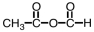

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
30
Phosphate esters are prepared from
A)phosphoric acid + alcohol.
B)phosphoric acid + carboxylic acid.
C)phosphoric acid + ester.
D)ester + phosphate.
A)phosphoric acid + alcohol.
B)phosphoric acid + carboxylic acid.
C)phosphoric acid + ester.
D)ester + phosphate.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following can react with alcohols to form esters?
A)sulfuric acid
B)carboxylic acid
C)nitric acid
D)more than one response is correct
A)sulfuric acid
B)carboxylic acid
C)nitric acid
D)more than one response is correct

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
32
The sodium salt of benzoic acid is used as
A)a food preservative.
B)an antacid.
C)a flavoring agent.
D)a soap.
A)a food preservative.
B)an antacid.
C)a flavoring agent.
D)a soap.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
33
The IUPAC name of the ester formed from methanol and benzoic acid is called
A)methyl benzoic acid.
B)methanol benzoate.
C)methyl benzoate.
D)benzyl methanoate.
A)methyl benzoic acid.
B)methanol benzoate.
C)methyl benzoate.
D)benzyl methanoate.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following materials is a product from the given reaction? 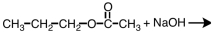
A)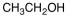
B)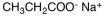
C)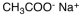
D)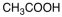
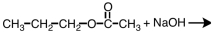
A)
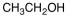
B)
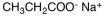
C)
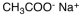
D)
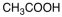

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
35
Which term correctly describes the following reaction? 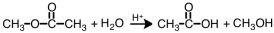
A)esterification
B)dissociation
C)hydrolysis
D)saponification

A)esterification
B)dissociation
C)hydrolysis
D)saponification

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
36
Saponification is a chemical reaction
A)that results in a soap.
B)due to the presence of a strong organic acid.
C)that results in an alcohol and a carboxylic acid.
D)that explains why soap does not wash off easily in hard water.
A)that results in a soap.
B)due to the presence of a strong organic acid.
C)that results in an alcohol and a carboxylic acid.
D)that explains why soap does not wash off easily in hard water.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
37
When a carboxylic acid reacts with alcohol,the organic product is a(n)____.
A)acetal
B)hemiacetal
C)ester
D)salt
A)acetal
B)hemiacetal
C)ester
D)salt

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
38
Which substance,when oxidized,results in the formation of this compound? 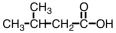
A)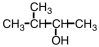
B)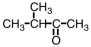
C)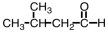
D)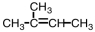
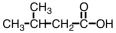
A)

B)

C)
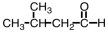
D)
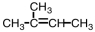

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
39
Which letter indicates the ester linkage in the following figure? 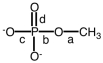
A)a
B)b
C)c
D)d

A)a
B)b
C)c
D)d

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
40
The pleasant,characteristic odor of fruit flavorings is often associated with the presence of
A)carboxylic acids.
B)carboxylic salts.
C)esters.
D)aldehydes.
A)carboxylic acids.
B)carboxylic salts.
C)esters.
D)aldehydes.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
41
Carboxylic acids dissociate in a manner shown in the following equation.Identify the conjugate base for the acid of the forward reaction. 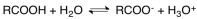
A)RCOOH
B)RCOO-
C)H2O
D)H3O+
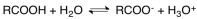
A)RCOOH
B)RCOO-
C)H2O
D)H3O+

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
42
How many mL of a 0.150 M solution of KOH would be required to titrate 25.0 mL of a 0.125 M solution of the following acid,which can be used as a standard in acid-base titrations? 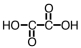
A)10.4 mL
B)30.0 mL
C)20.8 mL
D)41.7 mL

A)10.4 mL
B)30.0 mL
C)20.8 mL
D)41.7 mL

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
43
Carboxylic acids dissociate as showed in the following equation.Based on this information,we know that 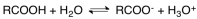
A)carboxylic acids are weak acids.
B)carboxylic acids are weak electrolytes
C)carboxylic acids will reduce the pH when added to water.
D)All of the above
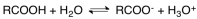
A)carboxylic acids are weak acids.
B)carboxylic acids are weak electrolytes
C)carboxylic acids will reduce the pH when added to water.
D)All of the above

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
44
Which compound could be used to produce the following compound on reaction with ethanol under appropriate conditions? 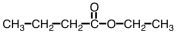
A)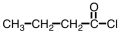
B)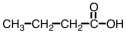
C)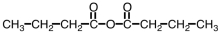
D)Any of them could be used.
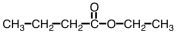
A)
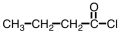
B)
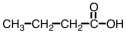
C)
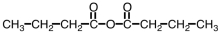
D)Any of them could be used.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
45
What is the IUPAC name for the compound shown below? 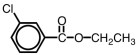
A)ethyl 2-chlorobenzoate
B)2-chloroethoxybenzoate
C)2-chloroethoxyphenolate
D)o-chloroethoxybenzoate

A)ethyl 2-chlorobenzoate
B)2-chloroethoxybenzoate
C)2-chloroethoxyphenolate
D)o-chloroethoxybenzoate

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
46
A common preservative found in many foods to prevent the growth of bacteria and mold is
A)sodium propanoate.
B)ethyl butanoate.
C)sodium stearate.
D)methyl salicylate.
A)sodium propanoate.
B)ethyl butanoate.
C)sodium stearate.
D)methyl salicylate.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following has the greatest solubility in water?
A)formic acid
B)propionic acid
C)acetic acid
D)all are equal
A)formic acid
B)propionic acid
C)acetic acid
D)all are equal

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following causes the noxious odor of rancid butter?
A)butyric acid
B)oxalic acid
C)lactic acid
D)stearic acid
A)butyric acid
B)oxalic acid
C)lactic acid
D)stearic acid

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
49
A wax is the product of the reaction between a fatty acid and fatty alcohol.As such,you would expect the resulting functional group to be which of the following? (Hint: The term fatty simply means that the carbon chains involved are both linear and at least 12 carbons long).
A)ether
B)anhydride
C)glycol
D)ester
A)ether
B)anhydride
C)glycol
D)ester

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
50
What would be produced as the result of a dehydration reaction between a carboxylic acid and an alcohol?
A)aldehyde
B)ester
C)acid anhydride
D)alkene
A)aldehyde
B)ester
C)acid anhydride
D)alkene

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following is the structure of 3-bromopropanoic acid?
A)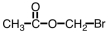
B)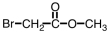
C)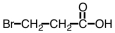
D)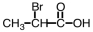
A)
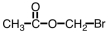
B)
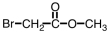
C)
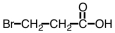
D)
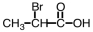

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
52
Reacting a carboxylic acid with a base will produce?
A)an ester
B)no reaction
C)a carboxylate salt
D)an anhydride
A)an ester
B)no reaction
C)a carboxylate salt
D)an anhydride

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following correctly shows what is produced as a result of increased oxidation of carbon?
A)alcohol > aldehyde > ketone > carboxylic acid
B)alkene > alcohol > aldehyde > carboxylic acid
C)ketone > alcohol > carboxylic acid > aldehyde
D)alcohol > alkene > ketone > aldehyde
A)alcohol > aldehyde > ketone > carboxylic acid
B)alkene > alcohol > aldehyde > carboxylic acid
C)ketone > alcohol > carboxylic acid > aldehyde
D)alcohol > alkene > ketone > aldehyde

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
54
Reacting a carboxylic acid with an alcohol will produce?
A)an ester
B)no reaction
C)a carboxylate salt
D)an anhydride
A)an ester
B)no reaction
C)a carboxylate salt
D)an anhydride

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
55
The following ion could be converted to the corresponding carboxylic acid by _____. 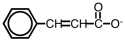
A)heating
B)adding an acid
C)adding water
D)adding a base
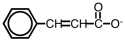
A)heating
B)adding an acid
C)adding water
D)adding a base

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
56
Alpha hydroxy acids can be found in which of the following?
A)apples
B)sour milk
C)sugar beets
D)all the choices
A)apples
B)sour milk
C)sugar beets
D)all the choices

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following sets of reactants could be used to produce a polyester?
A)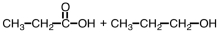
B)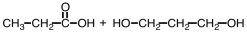
C)
D)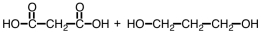
A)
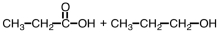
B)
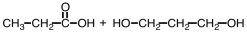
C)
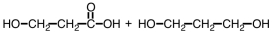
D)
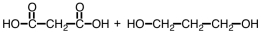

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following statements about carboxylic acids is true?
A)All are weak acids.
B)Produce hydrogen ions in water.
C)Will react with strong bases.
D)All of the choices are true.
A)All are weak acids.
B)Produce hydrogen ions in water.
C)Will react with strong bases.
D)All of the choices are true.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
59
If an intermediate in metabolism is a diester of phosphoric acid,it would have which general formula? (Note: R represents a generic alkyl chain.)
A)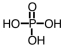
B)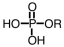
C)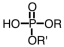
D)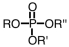
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following is structure of bromomethyl ethanoate?
A)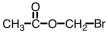
B)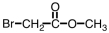
C)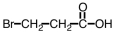
D)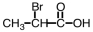
A)
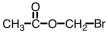
B)
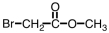
C)
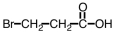
D)
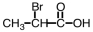

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
61
Carboxylic acids react readily with sodium hydroxide to form salts.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
62
Pure liquid carboxylic acids are strongly hydrogen bonded even in the absence of water.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
63
The other product in the formation of an ester from an acid and an alcohol is water.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
64
At pH 2,the predominant form of a carboxylic acid is the carboxylate anion.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
65
The carboxyl group found in carboxylic acids must be on a terminal carbon.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
66
The simplest of the carboxylic acids is formic acid.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
67
Citric acid is a tricarboxylic acid.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
68
Esters contain a carbonyl group attached directly to two carbons.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
69
Because carboxylic acids can have more than one carboxyl carbon,they are among the more acidic compounds found in the study of chemistry.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
70
Decanoic acid would likely be water soluble.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
71
Even though aspirin is named acetylsalicylic acid,aspirin contains the ester configuration due to the chemical reaction of a carboxyl group of the salicylic acid and acetic acid.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
72
Aldehydes are usually higher boiling than carboxylic acids with the same number of carbon atoms.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
73
Esters are formed when an alcohol and a carboxylic acid react by means of hydrolysis.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
74
An ester is equivalent to the inorganic term salt.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
75
Butyric acid is composed of a molecule that is small enough to evaporate from the liquid state at room temperature and,therefore,may have a scent.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
76
Some alpha-hydroxy acids are used in cosmetics as an exfoliating agent to remove dead skin.Which of the following compounds would be considered an alpha hydroxy acid?
A)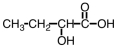
B)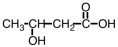
C)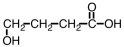
D)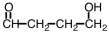
A)

B)
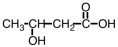
C)
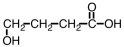
D)
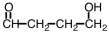

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
77
Carboxylic acids are not polar and are expected to have a relatively high boiling point.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
78
Carboxylic acids are weaker acids than HCl.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
79
Carboxylic acids are stronger acids than water.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
80
Nitroglycerin is a compound that is formed by the reaction of three molecules of nitric acid with a molecule of glycerol,and is an ester.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck


