Deck 19: Spontaneous Change: Entropy and Free Energy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/101
Play
Full screen (f)
Deck 19: Spontaneous Change: Entropy and Free Energy
1
A spontaneous reaction is one that must have a negative value of ΔH.
False
2
If a process is spontaneous, the reverse process is nonspontaneous.
True
3
ΔG∘ is independent of temperature.
False
4
Choose the correct statements concerning entropy. I) As two gasses mix, ΔS is positive.
II) Entropy is a thermodynamic property related to the degree of disorder.
III) As temperature in a gas decreases, ΔS is positive.
IV) Molecules in the liquid state have higher entropy than molecules in the gaseous state.
A) I and III
B) I, II, III
C) I and II
D) I, II, IV
E) II and III
II) Entropy is a thermodynamic property related to the degree of disorder.
III) As temperature in a gas decreases, ΔS is positive.
IV) Molecules in the liquid state have higher entropy than molecules in the gaseous state.
A) I and III
B) I, II, III
C) I and II
D) I, II, IV
E) II and III

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
5
A nonspontaneous reaction can be made to occur by coupling it with a spontaneous reaction to form an overall spontaneous reaction.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following processes would result in a decrease in system entropy?
A) melting of an ice cube
B) sublimation of a moth ball
C) evaporation of a puddle of gasoline
D) a glass of cool lemonade warming in the sun
E) condensation of water vapor on a cold windshield
A) melting of an ice cube
B) sublimation of a moth ball
C) evaporation of a puddle of gasoline
D) a glass of cool lemonade warming in the sun
E) condensation of water vapor on a cold windshield

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
7
ΔG is positive for a spontaneous reaction.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
8
Entropy is related to the way in which the energy of a system is distributed among the available microscopic energy levels.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
9
A spontaneous process will occur only if a some external action is continually applied.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
10
Indicate the statement(s) which are true for the process: Al+3(aq) + 3 OH-(aq) → Al(OH)3(s)
If it occurs in a closed container.
I. ΔS increases because the final molecule is more complicated.
II. Entropy decreases because the product is in the solid phase.
III. The two ions achieve a high degree of order as they crystalize, therefore ΔS is positive.
IV. Entropy of the system is unchanged because the system is sealed and at a constant temperature.
A) I and II
B) I and III
C) II only
D) I, II, IV
E) I and IV
If it occurs in a closed container.
I. ΔS increases because the final molecule is more complicated.
II. Entropy decreases because the product is in the solid phase.
III. The two ions achieve a high degree of order as they crystalize, therefore ΔS is positive.
IV. Entropy of the system is unchanged because the system is sealed and at a constant temperature.
A) I and II
B) I and III
C) II only
D) I, II, IV
E) I and IV

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
11
Nonspontaneous reactions and spontaneous reactions cannot be coupled.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
12
The entropy of a pure perfect crystal at 25 K is zero.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
13
The entropy of a system always increases for a spontaneous process.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
14
Which material has the largest entropy?
A) cannot be determined
B) pure water
C) powdered sugar
D) salt water
E) crystalline salt
A) cannot be determined
B) pure water
C) powdered sugar
D) salt water
E) crystalline salt

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
15
A spontaneous process:
A) will happen quickly.
B) releases large amounts of energy.
C) requires an external action in order to begin reacting.
D) will continue on its own once begun.
E) is never endothermic.
A) will happen quickly.
B) releases large amounts of energy.
C) requires an external action in order to begin reacting.
D) will continue on its own once begun.
E) is never endothermic.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following has the highest entropy?
A) 1 mole of liquid water at 30°C
B) 1 mole of water vapor at 30°C
C) 1 mole of regular ice at -10°C
D) 1 mole of "dry ice" at -10°C
E) 1 mole of water under 10 atm of pressure at -10°C
A) 1 mole of liquid water at 30°C
B) 1 mole of water vapor at 30°C
C) 1 mole of regular ice at -10°C
D) 1 mole of "dry ice" at -10°C
E) 1 mole of water under 10 atm of pressure at -10°C

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
17
Standard Gibbs energy of formation requires the reactants be compounds in their standard state.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following statements are true?
I. Liquids have more entropy than their solids.
II. Solutions have more entropy than the solids dissolved.
III. Gases and their liquids have equal entropy.
IV. Gases have less entropy than their solids.
V. Entropy of a substance increases as its temperature increases.
A) II), III), and V)
B) I), III), and V)
C) I), IV), and V)
D) I), II), and V)
E) II), IV) and V)
I. Liquids have more entropy than their solids.
II. Solutions have more entropy than the solids dissolved.
III. Gases and their liquids have equal entropy.
IV. Gases have less entropy than their solids.
V. Entropy of a substance increases as its temperature increases.
A) II), III), and V)
B) I), III), and V)
C) I), IV), and V)
D) I), II), and V)
E) II), IV) and V)

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
19
Find correct statements.
I. A spontaneous process is a process that occurs in a system left to itself.
II. A nonspontaneous process will not occur unless some external force is applied.
III. If a reaction is spontaneous, the reverse is also spontaneous.
IV. Only spontaneous processes occur naturally.
V. Entropy is inversely proportional to the degree of randomness.
A) I), II) and V)
B) II), III), and IV)
C) I), II), and IV)
D) I), III) and IV)
E) I), IV), and V)
I. A spontaneous process is a process that occurs in a system left to itself.
II. A nonspontaneous process will not occur unless some external force is applied.
III. If a reaction is spontaneous, the reverse is also spontaneous.
IV. Only spontaneous processes occur naturally.
V. Entropy is inversely proportional to the degree of randomness.
A) I), II) and V)
B) II), III), and IV)
C) I), II), and IV)
D) I), III) and IV)
E) I), IV), and V)

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
20
A zero ΔG means the system is at equilibrium.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
21
If ΔG < 0 for a reaction, then the reaction is said to be:
A) endothermic
B) reversible
C) spontaneous
D) exothermic
E) fast
A) endothermic
B) reversible
C) spontaneous
D) exothermic
E) fast

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following combinations of signs for ΔH and ΔS will always result in a reaction being nonspontaneous?
A) ΔH+, ΔS-
B) ΔH-, ΔS+
C) ΔH-, ΔS-
D) ΔH+, ΔS+
E) cannot determine without temperature
A) ΔH+, ΔS-
B) ΔH-, ΔS+
C) ΔH-, ΔS-
D) ΔH+, ΔS+
E) cannot determine without temperature

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following has the largest molar entropy?
A) I2(g)
B) Xe(g)
C) H2(g)
D) He(g)
A) I2(g)
B) Xe(g)
C) H2(g)
D) He(g)

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
24
Choose the INCORRECT statement.
A) The third law of thermodynamics states that the entropy of a pure crystal at 298 K is zero.
B) ΔS° = Σ (ν S°) products - Σ (ν S°) reactants.
C) The activity of pure liquids or pure solids is 1.
D) ΔG° = -RT ln Keq.
E) ΔG° = ΔH° - TΔS°.
A) The third law of thermodynamics states that the entropy of a pure crystal at 298 K is zero.
B) ΔS° = Σ (ν S°) products - Σ (ν S°) reactants.
C) The activity of pure liquids or pure solids is 1.
D) ΔG° = -RT ln Keq.
E) ΔG° = ΔH° - TΔS°.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
25
For an exothermic reaction to be nonspontaneous at high temperatures, the enthalpy must be ________ while the entropy is ________.
A) positive, also positive
B) positive, negative
C) a relatively small negative value, also negative
D) a relatively large negative value, positive
E) a very large negative value, also negative
A) positive, also positive
B) positive, negative
C) a relatively small negative value, also negative
D) a relatively large negative value, positive
E) a very large negative value, also negative

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
26
If ΔG is positive for a certain reaction, then:
A) the reaction is spontaneous.
B) the reverse of the reaction is spontaneous.
C) the system is in equilibrium.
D) one would need to know the Kelvin temperature to determine spontaneity.
E) there would be no reaction possible for a negative value.
A) the reaction is spontaneous.
B) the reverse of the reaction is spontaneous.
C) the system is in equilibrium.
D) one would need to know the Kelvin temperature to determine spontaneity.
E) there would be no reaction possible for a negative value.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
27
The normal melting and boiling points of SO2 are 198 and 263 K, respectively. For a plot of the standard molar entropy of SO2 versus temperature, which of the following is INCORRECT?
A) At 263 K, S° is constant.
B) S° increases with increasing temperature.
C) S° equals zero at 0 K.
D) At 198 K, S° increases by the value of △Sfusion.
A) At 263 K, S° is constant.
B) S° increases with increasing temperature.
C) S° equals zero at 0 K.
D) At 198 K, S° increases by the value of △Sfusion.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
28
The maximum quantity of energy available for useful work is:
A) constant
B) Gibbs energy
C) the entropy
D) the internal energy
E) the enthalpy
A) constant
B) Gibbs energy
C) the entropy
D) the internal energy
E) the enthalpy

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
29
The change in Gibbs energy for a reaction:
A) = ΔH - TΔS
B) = Q (heat)
C) = ΔS + TΔH
D) = ΔS - TΔH
E) = ΔH + TΔS
A) = ΔH - TΔS
B) = Q (heat)
C) = ΔS + TΔH
D) = ΔS - TΔH
E) = ΔH + TΔS

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
30
The Gibbs energy change for a reaction is -298 kJ. The reaction is therefore:
A) exothermic
B) irreversible
C) spontaneous
D) endothermic
E) nonspontaneous
A) exothermic
B) irreversible
C) spontaneous
D) endothermic
E) nonspontaneous

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following best expresses the increased degree of randomness associated with melting and sublimation, respectively?
A) ΔSfus - ΔSsub = 0
B) ΔSfus = 144.3 J/mol-K, ΔSsub = 9.0 J/mol-K
C) ΔSfus = 9.0 J/mol-K, ΔSsub = -144.3 J/mol-K
D) ΔH for both processes is zero
E) ΔSfus = 9.0 J/mol-K, ΔSsub = 144.3 J/mol-K
A) ΔSfus - ΔSsub = 0
B) ΔSfus = 144.3 J/mol-K, ΔSsub = 9.0 J/mol-K
C) ΔSfus = 9.0 J/mol-K, ΔSsub = -144.3 J/mol-K
D) ΔH for both processes is zero
E) ΔSfus = 9.0 J/mol-K, ΔSsub = 144.3 J/mol-K

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following relations is true for the molar entropy of sublimation of a substance?
A) ΔSsub = ΔSfusion + ΔSvaporization
B) ΔSsub < ΔSvaporization
C) ΔSsub = ΔSfusion - ΔSvaporization
D) ΔSsub < (ΔSfusion + ΔSvaporization)
A) ΔSsub = ΔSfusion + ΔSvaporization
B) ΔSsub < ΔSvaporization
C) ΔSsub = ΔSfusion - ΔSvaporization
D) ΔSsub < (ΔSfusion + ΔSvaporization)

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following substances under equal conditions and in the same phase has the greatest molar entropy?
A) NO
B) NO2
C) N2O3
D) N2O4
E) N2O5
A) NO
B) NO2
C) N2O3
D) N2O4
E) N2O5

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
34
In a sealed container, the rate of dissolving is equal to the rate of crystallization. Therefore we would expect:
A) ΔG < 0
B) ΔG > 0
C) ΔG = 0
D) ΔS = 0
E) must know ΔH to determine
A) ΔG < 0
B) ΔG > 0
C) ΔG = 0
D) ΔS = 0
E) must know ΔH to determine

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
35
A reaction is spontaneous if:
I. ΔG is a negative value
II. both enthalpy and entropy increase
III. ΔH is negative and ΔS is positive
IV. both enthalpy and entropy decrease
V. ΔH is positive and ΔS is negative
A) I and III
B) I and II
C) II and V
D) III and IV
E) II and IV
I. ΔG is a negative value
II. both enthalpy and entropy increase
III. ΔH is negative and ΔS is positive
IV. both enthalpy and entropy decrease
V. ΔH is positive and ΔS is negative
A) I and III
B) I and II
C) II and V
D) III and IV
E) II and IV

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
36
The following reaction is exothermic. 2N2O(g) → 2N2(g) + O2(g)
This means the reaction:
A) will be spontaneous at all temperatures
B) will be spontaneous only at high temperature
C) will be spontaneous only at low temperatures
D) is not spontaneous at any temperature
This means the reaction:
A) will be spontaneous at all temperatures
B) will be spontaneous only at high temperature
C) will be spontaneous only at low temperatures
D) is not spontaneous at any temperature

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
37
The following reaction is endothermic. 2NH3(g) → N2(g) + 3H2(g)
This means the reaction:
A) will be spontaneous at high temperature
B) will be spontaneous at low temperature
C) is not spontaneous at any temperature
D) is spontaneous at all temperatures
This means the reaction:
A) will be spontaneous at high temperature
B) will be spontaneous at low temperature
C) is not spontaneous at any temperature
D) is spontaneous at all temperatures

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
38
The change in Gibbs energy of a reaction:
A) = work
B) predicts speed
C) = ΔH + TΔS
D) depends on the standard state chosen
E) tells us if the reaction is spontaneous or not
A) = work
B) predicts speed
C) = ΔH + TΔS
D) depends on the standard state chosen
E) tells us if the reaction is spontaneous or not

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
39
Choose the INCORRECT statement.
A) One form of the second law of thermodynamics is all spontaneous processes produce an increase in the entropy of the universe.
B) Gibbs energy is defined by: G = H - TS.
C) If ΔG < 0, the process is spontaneous.
D) If ΔG > 0, the process is nonspontaneous.
E) If ΔG = 0, the process is spontaneous.
A) One form of the second law of thermodynamics is all spontaneous processes produce an increase in the entropy of the universe.
B) Gibbs energy is defined by: G = H - TS.
C) If ΔG < 0, the process is spontaneous.
D) If ΔG > 0, the process is nonspontaneous.
E) If ΔG = 0, the process is spontaneous.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
40
The fact that the entropies of vaporization for liquids which exhibit hydrogen bonding are greater than the 87 J/(mol ∙ K) which is expected of non-polar liquids is an exception to:
A) the Gibb's Energy Rule
B) the Third Law of Thermodynamics
C) the Clausius-Clapyeron Rule
D) the Second Law of Thermodynamics
E) Trouton's Rule
A) the Gibb's Energy Rule
B) the Third Law of Thermodynamics
C) the Clausius-Clapyeron Rule
D) the Second Law of Thermodynamics
E) Trouton's Rule

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
41
The equilibrium constant for the reaction below is 7.2 x 10-4 at 298 K and 1 atm. HNO2(aq) + H2O(l) ⇌ NO2-(aq) + H3O+(aq)
When [HNO2(aq)] = 1.0 M and [NO2-(aq)] = [H3O+(aq)] = 1.0 x 10-5 M, calculate ΔG.
A) -39.1 kJ/mol
B) +17.9 kJ/mol
C) -17.9 kJ/mol
D) +39.1 kJ/mol
When [HNO2(aq)] = 1.0 M and [NO2-(aq)] = [H3O+(aq)] = 1.0 x 10-5 M, calculate ΔG.
A) -39.1 kJ/mol
B) +17.9 kJ/mol
C) -17.9 kJ/mol
D) +39.1 kJ/mol

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
42
For the reaction: CO(g) + 2 H2(g) → CH3OH(g) Kp = 91.4 at 350 K and Kp = 2.05 × 10-4 at 298 K.
What is the value of ΔH°?
A) 49.9 kJ/mol
B) 2.08 × 103 kJ/mol
C) 3.74 × 10-2 kJ/mol
D) 217 kJ/mol
E) 446 kJ/mol
What is the value of ΔH°?
A) 49.9 kJ/mol
B) 2.08 × 103 kJ/mol
C) 3.74 × 10-2 kJ/mol
D) 217 kJ/mol
E) 446 kJ/mol

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
43
For the reaction, CaCO3(s) → CaO(s) + CO2(g) S° (J/mol K) 88.70 39.75 213.6
What is ΔS°rxn?
A) 262.5 J/mol ∙ K
B) -85.1 J/mol ∙ K
C) -164.7 J/mol ∙ K
D) 164.7 J/mol ∙ K
E) 85.1 J/mol ∙ K
What is ΔS°rxn?
A) 262.5 J/mol ∙ K
B) -85.1 J/mol ∙ K
C) -164.7 J/mol ∙ K
D) 164.7 J/mol ∙ K
E) 85.1 J/mol ∙ K

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
44
Consider the reaction: N2(g) + 3 X2(g) → 2 NX3(g)
△H° 0.0 0.0 -43 kJ/mol
S° 192 210 172 J/(mol∙K)
What is Keq for this reaction at 591 K?
A) 2.3 × 1017
B) 4.3 × 10-18
C) 1.04
D) 0.96
E) 132
△H° 0.0 0.0 -43 kJ/mol
S° 192 210 172 J/(mol∙K)
What is Keq for this reaction at 591 K?
A) 2.3 × 1017
B) 4.3 × 10-18
C) 1.04
D) 0.96
E) 132

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
45
Predict whether ΔS is positive or negative for the following process: H2O(g) → H2O(s)
A) negative
B) positive
C) There is not enough information to determine.
D) ΔS doesn't change.
E) ΔS changes the same on both sides of the equation.
A) negative
B) positive
C) There is not enough information to determine.
D) ΔS doesn't change.
E) ΔS changes the same on both sides of the equation.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
46
Choose the INCORRECT statement.
A) The van't Hoff equation is ln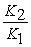 =
=
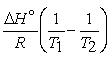 .
.
B) Keq is independent of temperature.
C) In a thermodynamic equilibrium constant expression, the activity of a gas is replaced by its partial pressure in atmosphere.
D) In a Keq expression, the activity of a solution is replaced by its molarity.
E) If ΔG = 0, the process is at equilibrium.
A) The van't Hoff equation is ln
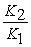 =
=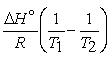 .
.B) Keq is independent of temperature.
C) In a thermodynamic equilibrium constant expression, the activity of a gas is replaced by its partial pressure in atmosphere.
D) In a Keq expression, the activity of a solution is replaced by its molarity.
E) If ΔG = 0, the process is at equilibrium.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
47
Order the following by increasing entropy. CO(g), COCl2(g), CO2(g), CaO(s)
A) CO2 < CO < CaO < COCl2
B) CaO < CO < CO2 < COCl2
C) COCl2 < CO < CaO < CO2
D) CO2 < CaO < COCl2 < CO
E) CO < CaO < COCl2 < CO2
A) CO2 < CO < CaO < COCl2
B) CaO < CO < CO2 < COCl2
C) COCl2 < CO < CaO < CO2
D) CO2 < CaO < COCl2 < CO
E) CO < CaO < COCl2 < CO2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following quantities is generally independent of temperature? I) ΔH
II) ΔS
III) ΔG
A) I only
B) II only
C) III only
D) I and II
E) I, II, and III
II) ΔS
III) ΔG
A) I only
B) II only
C) III only
D) I and II
E) I, II, and III

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
49
Predict whether ΔS is positive or negative for the following process: 2 Cl2O7(g) → 2 Cl2(g) + 7 O2(g)
A) negative
B) positive
C) There is not enough information to determine.
D) ΔS doesn't change.
E) ΔS changes the same on both sides of the equation.
A) negative
B) positive
C) There is not enough information to determine.
D) ΔS doesn't change.
E) ΔS changes the same on both sides of the equation.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
50
For the reaction PCl5(g) → PCl3(g) + Cl2(g) at 298 K, Keq = 1.87 × 10-7, ΔS° = 1.8192 J/(mol ∙K), what is ΔG° and is the reaction spontaneous?
A) 3.84 × 104 kJ/mol, no
B) 7.68 kJ/mol, no
C) -7.68 kJ/mol, yes
D) 38.4 kJ/mol, no
E) -38.4 kJ/mol, yes
A) 3.84 × 104 kJ/mol, no
B) 7.68 kJ/mol, no
C) -7.68 kJ/mol, yes
D) 38.4 kJ/mol, no
E) -38.4 kJ/mol, yes

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
51
If the vapor pressure of water in an open system at 25°C is 23.8 mmHg, what is ΔG for the reaction below at 25°C? H2O(l) → H2O(g, 23.8 mmHg)
A) 0 kJ/mol
B) -8.58 kJ/mol
C) +8.58 kJ/mol
D) -0.720 kJ/mol
A) 0 kJ/mol
B) -8.58 kJ/mol
C) +8.58 kJ/mol
D) -0.720 kJ/mol

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
52
For Cl2O(g) + 3/2 O2(g) → 2 ClO2 △H° = 126 kJ/mol, and ΔS° = -74.9 J/(mol∙deg) at 377°C. What is Keq?
A) 0.97
B) 6.12 × 10-7
C) 4.27 × 10-22
D) 9.17 × 10-15
E) 1.07 × 1014
A) 0.97
B) 6.12 × 10-7
C) 4.27 × 10-22
D) 9.17 × 10-15
E) 1.07 × 1014

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
53
Choose the INCORRECT statement about coupled reactions.
A) The usual practice of coupling reactions is to join two nonspontaneous reactions.
B) The usual reason to couple reactions is to produce an overall spontaneous reaction.
C) The reaction added to a nonspontaneous reaction needs to be spontaneous.
D) The reaction added to a nonspontaneous reaction needs to be more spontaneous than the original reaction is nonspontaneous.
E) One of the coupled reactions has a negative ΔG, the other a positive ΔG.
A) The usual practice of coupling reactions is to join two nonspontaneous reactions.
B) The usual reason to couple reactions is to produce an overall spontaneous reaction.
C) The reaction added to a nonspontaneous reaction needs to be spontaneous.
D) The reaction added to a nonspontaneous reaction needs to be more spontaneous than the original reaction is nonspontaneous.
E) One of the coupled reactions has a negative ΔG, the other a positive ΔG.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
54
For the reaction, CO(g) + 2H2(g) → CH3OH(g) S° (J/mol K) 197.6 130.6 239.7
What is ΔS°rxn?
A) -88.5 J/mol ∙ K
B) -176.7 J/mol ∙ K
C) 219 J/mol ∙ K
D) 176.7 J/mol ∙ K
E) -219.1 J/mol ∙ K
What is ΔS°rxn?
A) -88.5 J/mol ∙ K
B) -176.7 J/mol ∙ K
C) 219 J/mol ∙ K
D) 176.7 J/mol ∙ K
E) -219.1 J/mol ∙ K

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
55
Predict whether ΔS is positive or negative for the following process: H2(g) + 1/2 O2(g) → H2O(g)
A) negative
B) positive
C) There is not enough information to determine.
D) ΔS doesn't change.
E) ΔS changes the same on both sides of the equation.
A) negative
B) positive
C) There is not enough information to determine.
D) ΔS doesn't change.
E) ΔS changes the same on both sides of the equation.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
56
Consider the reaction of 25.0 mL of 0.20 M AgNO3(aq) with 25.0 mL of 0.20 M NaBr(aq) to form AgBr(s) at 25°C. What is ΔG for this reaction? The Ksp of AgBr is 5.0 x 10-13 at 25°C.
A) -58.8 kJ/mol
B) -70.2 kJ/mol
C) +58.8 kJ/mol
D) +70.2 kJ/mol
A) -58.8 kJ/mol
B) -70.2 kJ/mol
C) +58.8 kJ/mol
D) +70.2 kJ/mol

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
57
For the reaction, N2O4(g) → 2 NO2(g) S° (J/mol K) 304.2 240.0
What is ΔS°rxn?
A) 544.2 J/mol ∙ K
B) -64.2 J/mol ∙ K
C) 175.8 J/mol ∙ K
D) -175.8 J/mol ∙ K
E) 64.2 J/mol ∙ K
What is ΔS°rxn?
A) 544.2 J/mol ∙ K
B) -64.2 J/mol ∙ K
C) 175.8 J/mol ∙ K
D) -175.8 J/mol ∙ K
E) 64.2 J/mol ∙ K

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the listed reactions would couple with the following reaction to produce silver? Ag2O(s) → 2 Ag(s) + 1/2 O(g) ΔG° = 11.2 kJ/mol
A) Ag(s) + 1/2 Br2 → AgBr(s) ΔG° = 96.9 kJ/mol
B) C + 1/2 O2 → CO ΔG° = -137.2 kJ/mol
C) N + 1/2 O2 → NO ΔG° = 149.3 kJ/mol
D) N + O2 → NO2 ΔG° = 51.3 kJ/mol
E) C + 2 S → CS2(l) ΔG° = 65.3 kJ/mol
A) Ag(s) + 1/2 Br2 → AgBr(s) ΔG° = 96.9 kJ/mol
B) C + 1/2 O2 → CO ΔG° = -137.2 kJ/mol
C) N + 1/2 O2 → NO ΔG° = 149.3 kJ/mol
D) N + O2 → NO2 ΔG° = 51.3 kJ/mol
E) C + 2 S → CS2(l) ΔG° = 65.3 kJ/mol

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following quantities for an element has a value of zero in the standard state? I) ΔH°f
II) ΔG°f
III) S°
A) I only
B) II only
C) III only
D) I and II
E) I, II, and III
II) ΔG°f
III) S°
A) I only
B) II only
C) III only
D) I and II
E) I, II, and III

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
60
For the vaporization of water in an open system at 25°C and 1 atm, which of the following is correct?
A) The reaction is entropy driven.
B) The reaction is enthalpy driven.
C) The reaction is not spontaneous.
D) ΔG°rxn = 0
A) The reaction is entropy driven.
B) The reaction is enthalpy driven.
C) The reaction is not spontaneous.
D) ΔG°rxn = 0

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
61
Calculate ΔG° for the reaction Cu(s) + H2O(g) → CuO(s) + H2(g) at 500K.  Cu(s) 0 33.3 H2O(g) -241.8 188.7
Cu(s) 0 33.3 H2O(g) -241.8 188.7
CuO(s) -155.2 43.5
H2(g) 0 130.6
A) 231.8 kJ
B) -135.4 kJ
C) -58.6 kJ
D) 110.6 kJ
E) 86.74 kJ
 Cu(s) 0 33.3 H2O(g) -241.8 188.7
Cu(s) 0 33.3 H2O(g) -241.8 188.7CuO(s) -155.2 43.5
H2(g) 0 130.6
A) 231.8 kJ
B) -135.4 kJ
C) -58.6 kJ
D) 110.6 kJ
E) 86.74 kJ

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
62
If the enthalpy of vaporization of chloromethane, CH3Cl, is 21.5 kJ/mol at the normal boiling point, 249 K, calculate ΔS°vap.
A) 86.3 J mol-1 K-1
B) 5.35 J mol-1 K-1
C) 11.6 J mol-1 K-1
D) 896 J mol-1 K-1
A) 86.3 J mol-1 K-1
B) 5.35 J mol-1 K-1
C) 11.6 J mol-1 K-1
D) 896 J mol-1 K-1

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
63
What is ΔG°rxn? 2 O3(g) → 3 O2(g)
ΔGf° (J/mol K) 163.2 0
A) 326.2 kJ
B) -326.4 kJ
C) -163.2 kJ
D) 163.2 kJ
E) 54.4 kJ
ΔGf° (J/mol K) 163.2 0
A) 326.2 kJ
B) -326.4 kJ
C) -163.2 kJ
D) 163.2 kJ
E) 54.4 kJ

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
64
For the reaction I2(s) + Cl2(g) → 2 ICl(g) ΔH = 36 kJ and ΔS = 158.8 J/K at 25 °C. Calculate ΔG for the process at 25 °C.
A) -11.3 kJ
B) -4730 kJ
C) -393 kJ
D) 32 kJ
E) 83.3 kJ
A) -11.3 kJ
B) -4730 kJ
C) -393 kJ
D) 32 kJ
E) 83.3 kJ

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
65
What is ΔG° at 25°C? CO2(g) → CO2(aq) ΔH° = -19.4 kJ ΔS° = 92.3 J/K
A) 2.1 kJ
B) -46.9 kJ
C) -17.1 kJ
D) -19.5 kJ
E) -21.7 kJ
A) 2.1 kJ
B) -46.9 kJ
C) -17.1 kJ
D) -19.5 kJ
E) -21.7 kJ

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
66
Consider the following reaction: 4 NH3(g) + 7 O2(g) → 4 NO2(g) + 6 H2O (l)
ΔG°f -16.7 0.0 51.8 -237.2 kJ/mol
What is ΔG° for this reaction in kJ?
A) -1282
B) -1149
C) -169
D) 169
E) 1149
ΔG°f -16.7 0.0 51.8 -237.2 kJ/mol
What is ΔG° for this reaction in kJ?
A) -1282
B) -1149
C) -169
D) 169
E) 1149

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
67
Consider the following reaction. S2Cl2(g) + 2 OCl2(g) → 2 SOCl2(g) + Cl2(g)
ΔG°f -32 97.9 -320 0.0 kJ/mol
What is ΔG° for this reaction in kJ?
A) -804
B) -476
C) -386
D) -413
E) -799
ΔG°f -32 97.9 -320 0.0 kJ/mol
What is ΔG° for this reaction in kJ?
A) -804
B) -476
C) -386
D) -413
E) -799

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
68
Consider the following reaction: C(g) + 2 H2(g) → CH4(g)
S° 5.69 130.58 186.19 J/mol∙deg
What is ΔS° for the above reaction in J/mol∙deg?
A) -49.9 J/mol∙deg
B) 49.9 J/mol∙deg
C) 80.7 J/mol∙deg
D) 115.2 J/mol∙deg
E) -80.7 J/mol∙deg
S° 5.69 130.58 186.19 J/mol∙deg
What is ΔS° for the above reaction in J/mol∙deg?
A) -49.9 J/mol∙deg
B) 49.9 J/mol∙deg
C) 80.7 J/mol∙deg
D) 115.2 J/mol∙deg
E) -80.7 J/mol∙deg

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
69
Calculate the entropy change for methanol at its normal boiling point of 64.5°C. ΔH° = 38.0 kJ/mol.
A) 0.589 kJ/mol ∙ K
B) 0.112 kJ/mol ∙ K
C) 589 kJ/mol ∙ K
D) 112 kJ/mol ∙ K
E) 0.589 × 10-2 kJ/mol ∙ K
A) 0.589 kJ/mol ∙ K
B) 0.112 kJ/mol ∙ K
C) 589 kJ/mol ∙ K
D) 112 kJ/mol ∙ K
E) 0.589 × 10-2 kJ/mol ∙ K

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
70
What is ΔG°rxn? CO(g) + 2 H2(g) → CH3OH(g)
ΔGf° (J/mol K) -137.2 0 -162.0
A) -24.8 kJ
B) -299.2 kJ
C) +24.8 kJ
D) 149.6 kJ
E) +299.2 kJ
ΔGf° (J/mol K) -137.2 0 -162.0
A) -24.8 kJ
B) -299.2 kJ
C) +24.8 kJ
D) 149.6 kJ
E) +299.2 kJ

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
71
Consider the reaction: 3 N2(g) + 2 O3(g) → 6 NO(g) ΔHf° 0.00 142.26 90.37 kJ/mol
S° 191.5 237.7 210.6 J/mol K
What is ΔG°rxn for this reaction in kJ at 500 K?
A) 93 kJ
B) 151 kJ
C) 365 kJ
D) -1.00 × 105 kJ
E) 441 kJ
S° 191.5 237.7 210.6 J/mol K
What is ΔG°rxn for this reaction in kJ at 500 K?
A) 93 kJ
B) 151 kJ
C) 365 kJ
D) -1.00 × 105 kJ
E) 441 kJ

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
72
Consider the following reaction: C3H4(g) + 2 H2(g) → C3H8(g)
S° 266.9 130.6 269.9 J/mol∙deg
What is ΔS° in J/mol∙deg?
A) 127.6 J/mol∙deg
B) -127.6 J/mol∙deg
C) 3.0 J/mol∙deg
D) -258.2 J/mol∙deg
E) -3.0 J/mol∙deg
S° 266.9 130.6 269.9 J/mol∙deg
What is ΔS° in J/mol∙deg?
A) 127.6 J/mol∙deg
B) -127.6 J/mol∙deg
C) 3.0 J/mol∙deg
D) -258.2 J/mol∙deg
E) -3.0 J/mol∙deg

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
73
Consider the following reaction: NH3(g) + HCl(g) → NH4Cl(s)
S° 192.51 186.69 94.56 J/mol∙deg
What is ΔS° for this reaction in J/mol∙deg?
A) -284.6 J/mol∙deg
B) 284.6 J/mol∙deg
C) -92.3 J/mol∙deg
D) 94.6 J/mol∙deg
E) 92.3 J/mol∙deg
S° 192.51 186.69 94.56 J/mol∙deg
What is ΔS° for this reaction in J/mol∙deg?
A) -284.6 J/mol∙deg
B) 284.6 J/mol∙deg
C) -92.3 J/mol∙deg
D) 94.6 J/mol∙deg
E) 92.3 J/mol∙deg

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
74
What is ΔG° at 25°C? CO(g) + 2 H2(g) → CH3OH(g) ΔH° = -90.7 kJ ΔS° = -221 J/K
A) -90.9 kJ
B) -24.8 kJ
C) -156.6 kJ
D) -96.2 kJ
E) -85.2 kJ
A) -90.9 kJ
B) -24.8 kJ
C) -156.6 kJ
D) -96.2 kJ
E) -85.2 kJ

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
75
What is ΔG°rxn? N2O4(g) → 2 NO2(g)
ΔGf° (J/mol K) 97.8 51.3
A) 149.1 kJ
B) -4.8 kJ
C) -46.5 kJ
D) 4.8 kJ
E) 46.5 kJ
ΔGf° (J/mol K) 97.8 51.3
A) 149.1 kJ
B) -4.8 kJ
C) -46.5 kJ
D) 4.8 kJ
E) 46.5 kJ

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
76
What is ΔG° at 25°C? N2O4(g) → 2 NO2(g) ΔH° = 58.03 kJ ΔS° = 176.7 J/K
A) 58.21 kJ
B) 62.45 kJ
C) 53.6 kJ
D) 5.37 kJ
E) 111 kJ
A) 58.21 kJ
B) 62.45 kJ
C) 53.6 kJ
D) 5.37 kJ
E) 111 kJ

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
77
What is ΔG° at 25 °C? CaCO3(s) → CaO(s) + CO2(g) ΔH° = 177.8 kJ ΔS° = 160.7 J/K
A) 1779 kJ
B) 173.8 kJ
C) 225.7 kJ
D) 181.8 kJ
E) 129.9 kJ
A) 1779 kJ
B) 173.8 kJ
C) 225.7 kJ
D) 181.8 kJ
E) 129.9 kJ

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
78
What is ΔG° at 25°C? 2O3(g) → 3 O2(g) ΔH° = -284 kJ ΔS° = 139 J/K
A) -145 kJ
B) -243 kJ
C) -325 kJ
D) -281 kJ
E) -287 kJ
A) -145 kJ
B) -243 kJ
C) -325 kJ
D) -281 kJ
E) -287 kJ

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
79
What is ΔG°rxn? CaCO3(s) → CaO(s) + CO2(g)
ΔGf° (J/mol K) -1128 -604 -394.4
A) 1338 kJ
B) 918 kJ
C) -130 kJ
D) -918 kJ
E) 130 kJ
ΔGf° (J/mol K) -1128 -604 -394.4
A) 1338 kJ
B) 918 kJ
C) -130 kJ
D) -918 kJ
E) 130 kJ

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
80
For the reaction, 2 O3(g) → 3 O2(g)
S° (J/mol K) 238.8 205.0
What is ΔS°rxn?
A) 137.4 J/mol K
B) -137.4 J/mol K
C) 33.8 J/mol K
D) 171.2 J/mol K
E) -33.8 J/mol K
S° (J/mol K) 238.8 205.0
What is ΔS°rxn?
A) 137.4 J/mol K
B) -137.4 J/mol K
C) 33.8 J/mol K
D) 171.2 J/mol K
E) -33.8 J/mol K

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck


