Deck 14: The Ideal Gas Law and Its Applications
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/45
Play
Full screen (f)
Deck 14: The Ideal Gas Law and Its Applications
1
For which state of matter represented in the choices is the following statement applicable?
"Equal volumes of different substances contain the same number of particles."
A)
B)
C)
D)This statemeny does not apply to anyof the states of matter.
"Equal volumes of different substances contain the same number of particles."
A)

B)

C)

D)This statemeny does not apply to anyof the states of matter.

2
Which of the following represents the molar volume of an ideal gas at 273K and 1 bar?
A)22.7 mL/mol
B)22.7 L/mol
C)22.4 L/mol
D)0.0821 L/mol
E)Different gases have different molar volumes under these conditions.
A)22.7 mL/mol
B)22.7 L/mol
C)22.4 L/mol
D)0.0821 L/mol
E)Different gases have different molar volumes under these conditions.
22.7 L/mol
3
What is the density of helium gas at 17°C and 773 torr?
A)0.171 g/L
B)2.91 g/L
C)5.85 g/L
D)1.30* 102 g/L
E)2.22* 103 g/L
A)0.171 g/L
B)2.91 g/L
C)5.85 g/L
D)1.30* 102 g/L
E)2.22* 103 g/L
0.171 g/L
4
How many grams of oxygen gas occupy 549 mL at 743 torr and 18°C?
A)0.0225 g
B)0.359 g
C)11.6 g
D)0.751 g
E)0.719 g
A)0.0225 g
B)0.359 g
C)11.6 g
D)0.751 g
E)0.719 g

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
5
Consider the algebraic expression a* b = c * d *
A)b = c = d
B)b c d
C)a = e
D)a e
E)a *e= b *c * d
A)b = c = d
B)b c d
C)a = e
D)a e
E)a *e= b *c * d

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
6
The STP density of a gas is 1.46 grams per liter.Calculate its molar mass.
A)0.0306 g/mol
B)2.49 * 104 g/mol
C)3.00 g/mol
D)33.1 g/mol
E)35.7 g/mol
A)0.0306 g/mol
B)2.49 * 104 g/mol
C)3.00 g/mol
D)33.1 g/mol
E)35.7 g/mol

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
7
The density of an unknown gas is 0.731 g/L at 20°C and 1.035 atm.What is the molar mass of the gas?
A)1.16 g/mol
B)17.0 g/mol
C)32.0 g/mol
D)88.1 g/mol
E)2.52 *103 g/mol
A)1.16 g/mol
B)17.0 g/mol
C)32.0 g/mol
D)88.1 g/mol
E)2.52 *103 g/mol

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
8
What volume is occupied by 0.0100 mole of carbon monoxide at 9°C and 0.973 atm?
A)0.00759 L
B)0.132 L
C)0.238 L
D)4.20 L
E)577 L
A)0.00759 L
B)0.132 L
C)0.238 L
D)4.20 L
E)577 L

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following is a mathematical expression of the Ideal Gas Law,using the symbols commonly assigned to the measurable properties of gases in the chemistry literature (k represents a constant)?
A)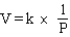
B)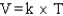
C)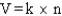
D)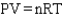
E)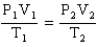
A)
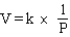
B)
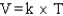
C)
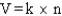
D)
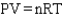
E)
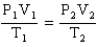

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
10
Consider the following representation of a gaseous chemical reaction. 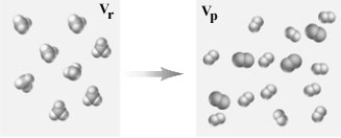 If carried out at constant temperature and pressure how would the volume in the container before the reaction (Vr)compare to that after the reaction (Vp)?
If carried out at constant temperature and pressure how would the volume in the container before the reaction (Vr)compare to that after the reaction (Vp)?
A)Vr = Vp
B)Vr = 2Vp
C)2Vr = Vp
D)3Vr = Vp
E)Vr = 3Vp
 If carried out at constant temperature and pressure how would the volume in the container before the reaction (Vr)compare to that after the reaction (Vp)?
If carried out at constant temperature and pressure how would the volume in the container before the reaction (Vr)compare to that after the reaction (Vp)?A)Vr = Vp
B)Vr = 2Vp
C)2Vr = Vp
D)3Vr = Vp
E)Vr = 3Vp

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
11
Consider the balloon shown below which has a volume of 0.34 L.  Determine the pressure in the balloon if the temperature is being held at 35°C and the balloon contains 0.0233 mol of an ideal gas.
Determine the pressure in the balloon if the temperature is being held at 35°C and the balloon contains 0.0233 mol of an ideal gas.
A)1.3 *103 atm
B)1.7 atm
C)0.58 atm
D)0.20 atm
 Determine the pressure in the balloon if the temperature is being held at 35°C and the balloon contains 0.0233 mol of an ideal gas.
Determine the pressure in the balloon if the temperature is being held at 35°C and the balloon contains 0.0233 mol of an ideal gas.A)1.3 *103 atm
B)1.7 atm
C)0.58 atm
D)0.20 atm

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
12
What is the common reference conditions for temperature and pressure (STP),respectively?
A)273 K,1 bar
B)100 °C,1 Pa
C)0 K,1 bar
D)273 K,1 atm
E)(0 K,1 bar)
A)273 K,1 bar
B)100 °C,1 Pa
C)0 K,1 bar
D)273 K,1 atm
E)(0 K,1 bar)

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
13
A gas has a density of 1.54 grams/liter at 1.14 atm and 15°C.Calculate the molar mass of the gas.
A)0.0313 g/mol
B)1.66 g/mol
C)31.9 g/mol
D)1.26 *103 g/mol
E)2.43 *104 g/mol
A)0.0313 g/mol
B)1.66 g/mol
C)31.9 g/mol
D)1.26 *103 g/mol
E)2.43 *104 g/mol

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
14
Determine the density of nitrogen gas at 21°C and 704 torr.
A)0.0384 g/L
B)0.537 g/L
C)0.816 g/L
D)0.934 g/L
E)1.08 g/L
A)0.0384 g/L
B)0.537 g/L
C)0.816 g/L
D)0.934 g/L
E)1.08 g/L

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
15
Calculate the number of moles in 3.13 L of dinitrogen monoxide at STP.
A)0.140 mol
B)7.16 mol
C)93.9 mol
D)70.1 mol
E)138 mol
A)0.140 mol
B)7.16 mol
C)93.9 mol
D)70.1 mol
E)138 mol

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following gases should have the largest density at STP?
A)HCN
B)Cl2
C)CH3CH2CH3
D)C4H10
E)The density cannot be predicted with the given data.
A)HCN
B)Cl2
C)CH3CH2CH3
D)C4H10
E)The density cannot be predicted with the given data.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
17
How does the volume of 3 *1022 hydrogen molecules compare with the volume of 3 * 1022 helium atoms,when both are measured at the same temperature and pressure?
A)The volume of hydrogen is twice the volume of helium
B)The volume of helium is twice the volume of hydrogen
C)The volume of hydrogen is four times the volume of helium
D)The volume of helium is four times the volume of hydrogen
E)Both volumes are the same
A)The volume of hydrogen is twice the volume of helium
B)The volume of helium is twice the volume of hydrogen
C)The volume of hydrogen is four times the volume of helium
D)The volume of helium is four times the volume of hydrogen
E)Both volumes are the same

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
18
Calculate the density of neon at 32°C and 0.676 atm.
A)7.17 * 10-4 g/L
B)6.84* 10-3 g/L
C)0.545 g/L
D)1.83 g/L
E)5.20 g/L
A)7.17 * 10-4 g/L
B)6.84* 10-3 g/L
C)0.545 g/L
D)1.83 g/L
E)5.20 g/L

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
19
Calculate the density of nitrogen dioxide at STP.
A)2.70 * 10-3 g/L
B)0.487 g/L
C)22.7 g/L
D)2.03 g/L
E)1.88 g/L
A)2.70 * 10-3 g/L
B)0.487 g/L
C)22.7 g/L
D)2.03 g/L
E)1.88 g/L

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
20
An unknown substance is vaporized at 200°C and 94.2 torr,under which conditions its density is 0.198 grams per liter.Find the molar mass.
A)0.0161 g/mol
B)0.0345 g/mol
C)0.0816 g/mol
D)26.2 g/mol
E)62.0 g/mol
A)0.0161 g/mol
B)0.0345 g/mol
C)0.0816 g/mol
D)26.2 g/mol
E)62.0 g/mol

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
21
How many moles are in 1.73 L of a gas if the molar volume is 37.5 L/mol?
A)0.0154 mol
B)0.0461 mol
C)21.7 mol
D)64.9 mol
E)22.4 mol
A)0.0154 mol
B)0.0461 mol
C)21.7 mol
D)64.9 mol
E)22.4 mol

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
22
A gas has a density of 1.19 g/L at STP.Which of the following could be this gas?
A)C4H10
B)C3H6
C)HCN
D)HI
E)OF2
A)C4H10
B)C3H6
C)HCN
D)HI
E)OF2

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
23
If 10.0 g of aluminum is consumed in the reaction 2 NaOH(aq)+ 2 Al(s)+ 6 H2O( 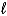 ) 2 NaAl(OH)4(aq)+ 3 H2(g),what volume of hydrogen,measured at -4°C and 0.88 atm,will be produced?
) 2 NaAl(OH)4(aq)+ 3 H2(g),what volume of hydrogen,measured at -4°C and 0.88 atm,will be produced?
A)6.2 L
B)12 L
C)14 L
D)16 L
E)19 L
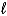 ) 2 NaAl(OH)4(aq)+ 3 H2(g),what volume of hydrogen,measured at -4°C and 0.88 atm,will be produced?
) 2 NaAl(OH)4(aq)+ 3 H2(g),what volume of hydrogen,measured at -4°C and 0.88 atm,will be produced?A)6.2 L
B)12 L
C)14 L
D)16 L
E)19 L

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
24
How many grams of hydrogen chloride are required to produce 12.0 liters of chlorine,measured at 1.22 atm and 19°C? 4 HCl + O2 2 Cl2 + 2 H2O
A)0.00837 g
B)0.0335 g
C)0.0224 g
D)44.5 g
E)11.1 g
A)0.00837 g
B)0.0335 g
C)0.0224 g
D)44.5 g
E)11.1 g

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
25
What volume of oxygen,measured at STP,can be produced by the decomposition of potassium chlorate if 20.0 g of the salt (KClO3)is completely decomposed into potassium chloride and oxygen gas?
A)1.83 L
B)2.44 L
C)3.66 L
D)2.14 L
E)5.55 L
A)1.83 L
B)2.44 L
C)3.66 L
D)2.14 L
E)5.55 L

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
26
Calculate the molar volume of nitrogen at 17°C and 5.27 atm.
A)0.211 L/mol
B)0.265 L/mol
C)4.52 L/mol
D)127 L/mol
E)3.43 * 103 L/mol
A)0.211 L/mol
B)0.265 L/mol
C)4.52 L/mol
D)127 L/mol
E)3.43 * 103 L/mol

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
27
What is the molar mass of a gas if 1.070 g of the gas occupies 264 mL at 100°C and 768 torr?
A)0.123 g/mol
B)0.162 g/mol
C)32.9 g/mol
D)64.0 g/mol
E)123 g/mol
A)0.123 g/mol
B)0.162 g/mol
C)32.9 g/mol
D)64.0 g/mol
E)123 g/mol

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
28
If the molar volume of a gas is 23.6 L/mol,how many moles are in 2.14 L?
A)50.5 mol
B)22.4 mol
C)11.0 mol
D)0.0907 mol
E)0.0198 mol
A)50.5 mol
B)22.4 mol
C)11.0 mol
D)0.0907 mol
E)0.0198 mol

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
29
Calculate the volume of 76.2 mole of propane gas,C3H8,if the molar volume is 55.0 L/mol.
A)1.39 L
B)61.1 L
C)1.85 * 105 L
D)2.39 * 10-4 L
E)4.19 * 103 L
A)1.39 L
B)61.1 L
C)1.85 * 105 L
D)2.39 * 10-4 L
E)4.19 * 103 L

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
30
How many liters of hydrogen,measured at 20°C and 750 torr,are required to react with 55.2 grams of aluminum oxide in the reaction Al2O3 + 3 H2 2 Al + 3 H2O?
A)36.6 L
B)1.62 L
C)2.70 L
D)0.0253 L
E)0.0520 L
A)36.6 L
B)1.62 L
C)2.70 L
D)0.0253 L
E)0.0520 L

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
31
Calculate the molar volume of a gas at 21°C and 3.73 atm.
A)0.462 L/mol
B)0.155 L/mol
C)351 L/mol
D)4.92*103 L/mol
E)6.47 L/mol
A)0.462 L/mol
B)0.155 L/mol
C)351 L/mol
D)4.92*103 L/mol
E)6.47 L/mol

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
32
Calculate the volume of hydrogen,measured at 19°C and 688 torr,that will be produced from 0.45 g of sodium in the reaction 2 Na(s)+ 2 H2O( 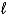 ) 2 NaOH(aq)+ H2(g).
) 2 NaOH(aq)+ H2(g).
A)1.9 *102 mL
B)2.1 * 102 mL
C)2.3 * 102 mL
D)2.6 * 102 mL
E)1.0 *103 mL
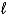 ) 2 NaOH(aq)+ H2(g).
) 2 NaOH(aq)+ H2(g).A)1.9 *102 mL
B)2.1 * 102 mL
C)2.3 * 102 mL
D)2.6 * 102 mL
E)1.0 *103 mL

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
33
What is the molar volume of a gas at 6°C and 975 torr?
A)17.9 L/mol
B)0.384 L/mol
C)0.0560 L/mol
D)0.0235 L/mol
E)5.05 * 10-4 L/mol
A)17.9 L/mol
B)0.384 L/mol
C)0.0560 L/mol
D)0.0235 L/mol
E)5.05 * 10-4 L/mol

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following statements is incorrect?
(i)The molar volume of a gas is the number of moles in one liter
(ii)The molar volume of a gas is variable depending on temperature and pressure
(iii)All gases have the same molar volume at the same temperature and pressure
A)All are correct
B)i only
C)ii only
D)iii only
E)ii and iii
(i)The molar volume of a gas is the number of moles in one liter
(ii)The molar volume of a gas is variable depending on temperature and pressure
(iii)All gases have the same molar volume at the same temperature and pressure
A)All are correct
B)i only
C)ii only
D)iii only
E)ii and iii

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
35
What is the volume of 0.0775 mole of nitrogen if the molar volume is 27.6 L/mol?
A)59.9 L
B)2.14 L
C)0.468 L
D)0.0786 L
E)2.81*10-3 L
A)59.9 L
B)2.14 L
C)0.468 L
D)0.0786 L
E)2.81*10-3 L

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
36
What is the molar volume of CO2 at 39°C and 652 torr?
A)0.0335 L/mol
B)0.0393 L/mol
C)3.73 L/mol
D)29.9 L/mol
E)1.32 *103 L/mol
A)0.0335 L/mol
B)0.0393 L/mol
C)3.73 L/mol
D)29.9 L/mol
E)1.32 *103 L/mol

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
37
Consider the reaction CH4(g)+ H2O(g) 3 H2(g)+ CO(g).If 2.25 L of methane,measured at STP,react,how many liters of hydrogen will be produced if it is also measured at STP?
A)0.094 L
B)0.75 L
C)0.84 L
D)2.25 L
E)6.75 L
A)0.094 L
B)0.75 L
C)0.84 L
D)2.25 L
E)6.75 L

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
38
The molar volume of a gas at a certain temperature and pressure is 35.1 L/mol.What is the volume of 0.233 mol of the gas?
A)6.64 * 10-3 L
B)8.18 L
C)151 L
D)6.64 * 10-2 L
E)0.818 L
A)6.64 * 10-3 L
B)8.18 L
C)151 L
D)6.64 * 10-2 L
E)0.818 L

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
39
Determine the STP volume of sulfur hexafluoride that will be produced from the reaction of 25.0 kg of sulfur in the reaction S( 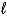 )+ 3 F2(g) SF6(g).
)+ 3 F2(g) SF6(g).
A)0.0348 kL
B)34.8 kL
C)0.0175 kL
D)17.7 kL
E)1.75 *104 kL
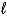 )+ 3 F2(g) SF6(g).
)+ 3 F2(g) SF6(g).A)0.0348 kL
B)34.8 kL
C)0.0175 kL
D)17.7 kL
E)1.75 *104 kL

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
40
Consider the reaction 2 NH3(g)+ CO2(g) H2NCONH2(s)+ H2O(g).If 93.5 kL of ammonia at STP is reacted with excess carbon dioxide,how many kilograms of urea will be formed?
A)124 kg
B)501 kg
C)1.05*103 kg
D)6.29 *104 kg
E)2.52*105 kg
A)124 kg
B)501 kg
C)1.05*103 kg
D)6.29 *104 kg
E)2.52*105 kg

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
41
A 399 mL sample of ethane at 45°C and 656 mm Hg is burned,producing carbon dioxide gas,which is captured,separated and cooled to 5°C.The pressure of the CO2 sample is adjusted to 7.50 *102 mm Hg.What is its volume? 2 C2H6(g)+ 7 O2(g) 4 CO2(g)+ 6 H2O(g)
A)153 mL
B)200 mL
C)261 mL
D)610 mL
E)798 mL
A)153 mL
B)200 mL
C)261 mL
D)610 mL
E)798 mL

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
42
Consider the reaction CO(g)+ 2 H2(g) CH3OH(g).If 15.0 L of hydrogen,measured at 22°C and 732 torr,reacts with excess carbon monoxide,what volume of methanol,measured at 345°C at 1.12*103 torr,will be produced?
A)2.34 L
B)5.48 L
C)10.3 L
D)24.0 L
E)41.1 L
A)2.34 L
B)5.48 L
C)10.3 L
D)24.0 L
E)41.1 L

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
43
Liquid butane burns according to the equation 2 C4H10( 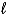 )+ 13 O2(g) 8 CO2(g)+ 10 H2O(g).How many grams of butane have burned if 3.39 L of carbon dioxide is produced at 34°C and 1.22 atm?
)+ 13 O2(g) 8 CO2(g)+ 10 H2O(g).How many grams of butane have burned if 3.39 L of carbon dioxide is produced at 34°C and 1.22 atm?
A)2.38 g
B)3.02 g
C)1.60 g
D)38.2 g
E)1.20 *103 g
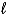 )+ 13 O2(g) 8 CO2(g)+ 10 H2O(g).How many grams of butane have burned if 3.39 L of carbon dioxide is produced at 34°C and 1.22 atm?
)+ 13 O2(g) 8 CO2(g)+ 10 H2O(g).How many grams of butane have burned if 3.39 L of carbon dioxide is produced at 34°C and 1.22 atm?A)2.38 g
B)3.02 g
C)1.60 g
D)38.2 g
E)1.20 *103 g

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
44
If 26.0 liters of methane,CH4,measured at 1.09 atm and 23.0?0°C,are converted to acetylene,C2H2,what volume of acetylene,measured at 0.941 atm and 185?0°C,will result? 2 CH4 C2H2 + 3 H2
A)9.73 L
B)17.4 L
C)23.3 L
D)93.2 L
E)0.0429 L
A)9.73 L
B)17.4 L
C)23.3 L
D)93.2 L
E)0.0429 L

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
45
An STP volume of 564 liters of oxygen is produced in the reaction 2 N2O5(g) 4 NO2(g)+ O2(g).What volume of nitrogen dioxide is also produced if it is measured at 25°C and 1.00 bar?
A)2.46 *103 L
B)1.87 * 106 L
C)2.07 * 103 L
D)154 L
E)5.64 * 104 L
A)2.46 *103 L
B)1.87 * 106 L
C)2.07 * 103 L
D)154 L
E)5.64 * 104 L

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck


