Deck 17: Equilibrium
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/56
Play
Full screen (f)
Deck 17: Equilibrium
1
Choose the correct equilibrium expression for the following reaction. A(s) + 2B(l) 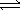 3C(aq) + 4D(aq)
3C(aq) + 4D(aq)
A)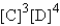
B)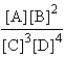
C)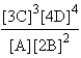
D)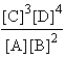
E) none of these
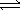 3C(aq) + 4D(aq)
3C(aq) + 4D(aq)A)
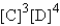
B)
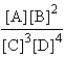
C)
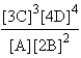
D)
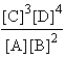
E) none of these
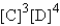
2
At equilibrium, the concentrations of all reactants and products are constant.
True
3
A catalyst is a substance that speeds up a reaction without being consumed.
True
4
A minimum energy called the activation energy is needed for a reaction to occur.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
5
Write the equilibrium expression for the following reaction: S(s) + O2(g)  SO2(g)
SO2(g)
A) K =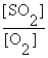
B) K =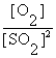
C) K =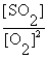
D) K =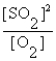
E) none of these
 SO2(g)
SO2(g)A) K =
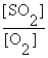
B) K =
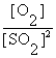
C) K =
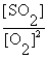
D) K =
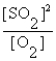
E) none of these

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
6
At equilibrium, the concentrations of all reactants and products are equal.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is an example of a heterogeneous equilibrium reaction?
A) N2O4(g) 2NO2(g)
2NO2(g)
B) H2(g) + F2(g) 2HF(g)
2HF(g)
C) C2H2(g) + 2Br2(g) C2H2Br4(g)
C2H2Br4(g)
D) N2(g) + 3H2(g) 2NH3(g)
2NH3(g)
E) MgO(s) + CO2(g) MgCO3(s)
MgCO3(s)
A) N2O4(g)
 2NO2(g)
2NO2(g)B) H2(g) + F2(g)
 2HF(g)
2HF(g)C) C2H2(g) + 2Br2(g)
 C2H2Br4(g)
C2H2Br4(g)D) N2(g) + 3H2(g)
 2NH3(g)
2NH3(g)E) MgO(s) + CO2(g)
 MgCO3(s)
MgCO3(s)
Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
8
The correct equilibrium expression for the reaction of sulfur dioxide gas with oxygen gas to produce sulfur trioxide gas is
A)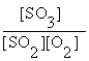
B)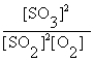
C)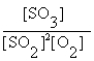
D)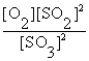
E) none of these
A)
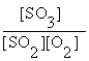
B)
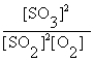
C)
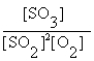
D)
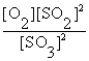
E) none of these

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
9
Write the equilibrium expression for the reaction
3O2(g) 2O3(g)
2O3(g)
3O2(g)
 2O3(g)
2O3(g)
Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
10
For the reaction F2(g) ![<strong>For the reaction F<sub>2</sub>(g) 2F(g) At a particular temperature, the concentrations at equilibrium were observed to be [F<sub>2</sub>] = 2.0 *10<sup>-</sup><sup>2</sup> mol/L and [F] = 2.0 * 10<sup>-</sup><sup>4</sup> mol/L. Calculate the value of the equilibrium constant from these data. (The units are deleted.)</strong> A) B) 1.7 C) 5.0 * 10<sup>5</sup> D) 2.0 * 10<sup>-</sup><sup>6</sup> E) none of these](https://storage.examlex.com/TB6421/11eab077_f7fc_fb9b_beed_855954edc022_TB6421_11.jpg) 2F(g)
2F(g)
At a particular temperature, the concentrations at equilibrium were observed to be [F2] = 2.0 *10-2 mol/L and [F] = 2.0 * 10-4 mol/L. Calculate the value of the equilibrium constant from these data. (The units are deleted.)
A)![<strong>For the reaction F<sub>2</sub>(g) 2F(g) At a particular temperature, the concentrations at equilibrium were observed to be [F<sub>2</sub>] = 2.0 *10<sup>-</sup><sup>2</sup> mol/L and [F] = 2.0 * 10<sup>-</sup><sup>4</sup> mol/L. Calculate the value of the equilibrium constant from these data. (The units are deleted.)</strong> A) B) 1.7 C) 5.0 * 10<sup>5</sup> D) 2.0 * 10<sup>-</sup><sup>6</sup> E) none of these](https://storage.examlex.com/TB6421/11eab077_f7fc_fb9c_beed_1f53c59ceed8_TB6421_11.jpg)
B) 1.7
C) 5.0 * 105
D) 2.0 * 10-6
E) none of these
![<strong>For the reaction F<sub>2</sub>(g) 2F(g) At a particular temperature, the concentrations at equilibrium were observed to be [F<sub>2</sub>] = 2.0 *10<sup>-</sup><sup>2</sup> mol/L and [F] = 2.0 * 10<sup>-</sup><sup>4</sup> mol/L. Calculate the value of the equilibrium constant from these data. (The units are deleted.)</strong> A) B) 1.7 C) 5.0 * 10<sup>5</sup> D) 2.0 * 10<sup>-</sup><sup>6</sup> E) none of these](https://storage.examlex.com/TB6421/11eab077_f7fc_fb9b_beed_855954edc022_TB6421_11.jpg) 2F(g)
2F(g)At a particular temperature, the concentrations at equilibrium were observed to be [F2] = 2.0 *10-2 mol/L and [F] = 2.0 * 10-4 mol/L. Calculate the value of the equilibrium constant from these data. (The units are deleted.)
A)
![<strong>For the reaction F<sub>2</sub>(g) 2F(g) At a particular temperature, the concentrations at equilibrium were observed to be [F<sub>2</sub>] = 2.0 *10<sup>-</sup><sup>2</sup> mol/L and [F] = 2.0 * 10<sup>-</sup><sup>4</sup> mol/L. Calculate the value of the equilibrium constant from these data. (The units are deleted.)</strong> A) B) 1.7 C) 5.0 * 10<sup>5</sup> D) 2.0 * 10<sup>-</sup><sup>6</sup> E) none of these](https://storage.examlex.com/TB6421/11eab077_f7fc_fb9c_beed_1f53c59ceed8_TB6421_11.jpg)
B) 1.7
C) 5.0 * 105
D) 2.0 * 10-6
E) none of these

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
11
The ____________ model explains why a reaction proceeds faster if the concentrations of the reacting molecules are increased.
A) atomic
B) gas law
C) collision
D) Lewis dot
E) none of these
A) atomic
B) gas law
C) collision
D) Lewis dot
E) none of these

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
12
Consider the reaction 2H2(g) + O2(g)  2H2O(g) at some equilibrium position. Using the following choices, indicate what will happen if the changes below are made.
2H2O(g) at some equilibrium position. Using the following choices, indicate what will happen if the changes below are made.
Some H2(g) is removed from the reaction vessel.
A) shifts to the left
B) shifts to the right
C) no change
 2H2O(g) at some equilibrium position. Using the following choices, indicate what will happen if the changes below are made.
2H2O(g) at some equilibrium position. Using the following choices, indicate what will happen if the changes below are made.Some H2(g) is removed from the reaction vessel.
A) shifts to the left
B) shifts to the right
C) no change

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
13
Consider the equilibrium shown here: CaCO3(s)  CaO(s) + CO2(g)
CaO(s) + CO2(g)
What would happen to the system if the total pressure were increased by adding CO2(g)?
A) Nothing would happen.
B) More CO2(g) would be produced.
C) The amount of CaO would increase.
D) The amount of CaCO3 would increase.
E) Equilibrium would shift to the right.
 CaO(s) + CO2(g)
CaO(s) + CO2(g)What would happen to the system if the total pressure were increased by adding CO2(g)?
A) Nothing would happen.
B) More CO2(g) would be produced.
C) The amount of CaO would increase.
D) The amount of CaCO3 would increase.
E) Equilibrium would shift to the right.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
14
Given the reaction A(g) + B(g)  C(g) + D(g). You have the gases A, B, C, and D at equilibrium. Upon adding gas A, the value of K
C(g) + D(g). You have the gases A, B, C, and D at equilibrium. Upon adding gas A, the value of K
A) increases because when A is added, more products are made, increasing the product-to-reactant ratio
B) decreases because A is a reactant, so the product-to-reactant ratio decreases
C) does not change because A does not figure into the product-to-reactant ratio
D) does not change as long as the temperature is constant
E) depends on whether the reaction is endothermic or exothermic
 C(g) + D(g). You have the gases A, B, C, and D at equilibrium. Upon adding gas A, the value of K
C(g) + D(g). You have the gases A, B, C, and D at equilibrium. Upon adding gas A, the value of KA) increases because when A is added, more products are made, increasing the product-to-reactant ratio
B) decreases because A is a reactant, so the product-to-reactant ratio decreases
C) does not change because A does not figure into the product-to-reactant ratio
D) does not change as long as the temperature is constant
E) depends on whether the reaction is endothermic or exothermic

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
15
Consider the equilibrium shown here: CaCO3(s)  CaO(s) + CO2(g)
CaO(s) + CO2(g)
What would happen to the system if the total pressure were increased by adding Ar(g)?
A) Nothing would happen.
B) More CO2(g) would be produced.
C) The amount of CaO would increase.
D) The amount of CaCO3 would increase.
E) Equilibrium would shift to the right.
 CaO(s) + CO2(g)
CaO(s) + CO2(g)What would happen to the system if the total pressure were increased by adding Ar(g)?
A) Nothing would happen.
B) More CO2(g) would be produced.
C) The amount of CaO would increase.
D) The amount of CaCO3 would increase.
E) Equilibrium would shift to the right.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
16
Consider the reaction 2H2(g) + O2(g)  2H2O(g) at some equilibrium position. Using the following choices, indicate what will happen if the changes below are made.
2H2O(g) at some equilibrium position. Using the following choices, indicate what will happen if the changes below are made.
Additional H2O(g) is injected into the reaction vessel.
A) shifts to the left
B) shifts to the right
C) no change
 2H2O(g) at some equilibrium position. Using the following choices, indicate what will happen if the changes below are made.
2H2O(g) at some equilibrium position. Using the following choices, indicate what will happen if the changes below are made.Additional H2O(g) is injected into the reaction vessel.
A) shifts to the left
B) shifts to the right
C) no change

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
17
For the reaction 2NO(g) + O2(g) ![<strong>For the reaction 2NO(g) + O<sub>2</sub>(g) 2NO<sub>2</sub>(g) At a certain temperature, the equilibrium concentrations were found to be [NO<sub>2</sub>] = 2.6 *10<sup>-</sup><sup>3</sup> M, [O<sub>2</sub>]= 1.0 *10<sup>-</sup><sup>2</sup> M, and [NO] = 2.0 * 10<sup>-</sup><sup>3</sup> M. Calculate the value of the equilibrium constant from these data (delete units).</strong> A) 1.7 * 10<sup>2</sup> B) 6.5 * 10<sup>4</sup> C) 1.3 *10<sup>2</sup> D) 5.9 * 10<sup>-</sup><sup>3</sup> E) none of these](https://storage.examlex.com/TB6421/11eab077_f7fd_22ad_beed_abf225a75377_TB6421_11.jpg) 2NO2(g)
2NO2(g)
At a certain temperature, the equilibrium concentrations were found to be [NO2] = 2.6 *10-3 M, [O2]= 1.0 *10-2 M, and [NO] = 2.0 * 10-3 M. Calculate the value of the equilibrium constant from these data (delete units).
A) 1.7 * 102
B) 6.5 * 104
C) 1.3 *102
D) 5.9 * 10-3
E) none of these
![<strong>For the reaction 2NO(g) + O<sub>2</sub>(g) 2NO<sub>2</sub>(g) At a certain temperature, the equilibrium concentrations were found to be [NO<sub>2</sub>] = 2.6 *10<sup>-</sup><sup>3</sup> M, [O<sub>2</sub>]= 1.0 *10<sup>-</sup><sup>2</sup> M, and [NO] = 2.0 * 10<sup>-</sup><sup>3</sup> M. Calculate the value of the equilibrium constant from these data (delete units).</strong> A) 1.7 * 10<sup>2</sup> B) 6.5 * 10<sup>4</sup> C) 1.3 *10<sup>2</sup> D) 5.9 * 10<sup>-</sup><sup>3</sup> E) none of these](https://storage.examlex.com/TB6421/11eab077_f7fd_22ad_beed_abf225a75377_TB6421_11.jpg) 2NO2(g)
2NO2(g)At a certain temperature, the equilibrium concentrations were found to be [NO2] = 2.6 *10-3 M, [O2]= 1.0 *10-2 M, and [NO] = 2.0 * 10-3 M. Calculate the value of the equilibrium constant from these data (delete units).
A) 1.7 * 102
B) 6.5 * 104
C) 1.3 *102
D) 5.9 * 10-3
E) none of these

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
18
Consider the general reaction aA + bB  cC + dD
cC + dD
Choose the correct equilibrium expression below.
A)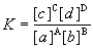
B)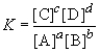
C)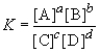
D)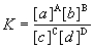
E)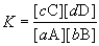
 cC + dD
cC + dDChoose the correct equilibrium expression below.
A)
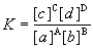
B)
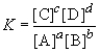
C)
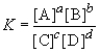
D)
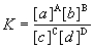
E)
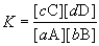

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is an example of a homogeneous equilibrium reaction?
A) 2CO(g) + O2(g) 2CO2(g)
2CO2(g)
B) PCl5(s) PCl3(l) + Cl2(g)
PCl3(l) + Cl2(g)
C) 2KClO3(s) 2KCl(s) + 3O2(g)
2KCl(s) + 3O2(g)
D) CaCO3(s) CaO(s) + CO2(g)
CaO(s) + CO2(g)
E) 6CO2(g) + 6H2O(g) C6H12O6(s) + 6O2(g)
C6H12O6(s) + 6O2(g)
A) 2CO(g) + O2(g)
 2CO2(g)
2CO2(g)B) PCl5(s)
 PCl3(l) + Cl2(g)
PCl3(l) + Cl2(g)C) 2KClO3(s)
 2KCl(s) + 3O2(g)
2KCl(s) + 3O2(g)D) CaCO3(s)
 CaO(s) + CO2(g)
CaO(s) + CO2(g)E) 6CO2(g) + 6H2O(g)
 C6H12O6(s) + 6O2(g)
C6H12O6(s) + 6O2(g)
Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
20
Consider the equilibrium shown here: CaCO3(s)  CaO(s) + CO2(g)
CaO(s) + CO2(g)
What would happen to the system if more CaCO3 were added?
A) More CaO would be produced.
B) The concentration of CO2(g) would decrease.
C) The amount of CaCO3 would decrease.
D) The pressure would increase.
E) Nothing would happen.
 CaO(s) + CO2(g)
CaO(s) + CO2(g)What would happen to the system if more CaCO3 were added?
A) More CaO would be produced.
B) The concentration of CO2(g) would decrease.
C) The amount of CaCO3 would decrease.
D) The pressure would increase.
E) Nothing would happen.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
21
Consider the following equilibrium: 2H2(g) + X2(g) ![<strong>Consider the following equilibrium: 2H<sub>2</sub>(g) + X<sub>2</sub>(g) 2H<sub>2</sub>X(g) + energy Addition of X<sub>2</sub> to this system at equilibrium</strong> A) will cause [H<sub>2</sub>] to decrease B) will cause [X<sub>2</sub>] to increase C) will cause [H<sub>2</sub>X] to increase D) will have no effect E) cannot possibly be carried out](https://storage.examlex.com/TB6421/11eab077_f800_a53c_beed_71eb34ce0308_TB6421_11.jpg) 2H2X(g) + energy
2H2X(g) + energy
Addition of X2 to this system at equilibrium
A) will cause [H2] to decrease
B) will cause [X2] to increase
C) will cause [H2X] to increase
D) will have no effect
E) cannot possibly be carried out
![<strong>Consider the following equilibrium: 2H<sub>2</sub>(g) + X<sub>2</sub>(g) 2H<sub>2</sub>X(g) + energy Addition of X<sub>2</sub> to this system at equilibrium</strong> A) will cause [H<sub>2</sub>] to decrease B) will cause [X<sub>2</sub>] to increase C) will cause [H<sub>2</sub>X] to increase D) will have no effect E) cannot possibly be carried out](https://storage.examlex.com/TB6421/11eab077_f800_a53c_beed_71eb34ce0308_TB6421_11.jpg) 2H2X(g) + energy
2H2X(g) + energyAddition of X2 to this system at equilibrium
A) will cause [H2] to decrease
B) will cause [X2] to increase
C) will cause [H2X] to increase
D) will have no effect
E) cannot possibly be carried out

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
22
Given the equation A(g)  B(g) + 2C(g). At a particular temperature, K = 1.4 *105.
B(g) + 2C(g). At a particular temperature, K = 1.4 *105.
-Which of the following is true when the equilibrium constant for a reaction is relatively large?
A) It will take a short time to reach equilibrium.
B) It will take a long time to reach equilibrium.
C) The equilibrium lies to the left.
D) The equilibrium lies to the right.
E) two of these
 B(g) + 2C(g). At a particular temperature, K = 1.4 *105.
B(g) + 2C(g). At a particular temperature, K = 1.4 *105.-Which of the following is true when the equilibrium constant for a reaction is relatively large?
A) It will take a short time to reach equilibrium.
B) It will take a long time to reach equilibrium.
C) The equilibrium lies to the left.
D) The equilibrium lies to the right.
E) two of these

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
23
Consider the following equilibrium: H2(g) + I2(s)  2HI(g)
2HI(g)
The equilibrium expression is
A)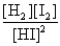
B)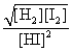
C)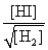
D)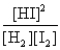
E)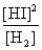
 2HI(g)
2HI(g)The equilibrium expression is
A)
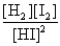
B)
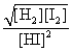
C)
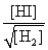
D)
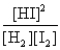
E)
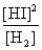

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
24
Given the equation A(g) 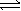 B(g) + 2C(g). At a particular temperature, K =
B(g) + 2C(g). At a particular temperature, K = 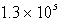 . If you mixed 1.4 mol B, 0.050 mol C, and 0.0050 mol A in a 1-liter container, in which direction would the reaction initially proceed?
. If you mixed 1.4 mol B, 0.050 mol C, and 0.0050 mol A in a 1-liter container, in which direction would the reaction initially proceed?
A) The reaction will proceed to the right.
B) The reaction will proceed to the left.
C) The mixture is at equilibrium.
D) More information is needed to answer the question.
 B(g) + 2C(g). At a particular temperature, K =
B(g) + 2C(g). At a particular temperature, K = 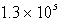 . If you mixed 1.4 mol B, 0.050 mol C, and 0.0050 mol A in a 1-liter container, in which direction would the reaction initially proceed?
. If you mixed 1.4 mol B, 0.050 mol C, and 0.0050 mol A in a 1-liter container, in which direction would the reaction initially proceed?A) The reaction will proceed to the right.
B) The reaction will proceed to the left.
C) The mixture is at equilibrium.
D) More information is needed to answer the question.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
25
Consider the following equilibrium: 2H2(g) + X2(g)  2H2X(g) + energy
2H2X(g) + energy
The equilibrium expression is
A)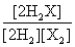
B)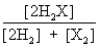
C)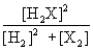
D)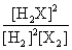
E) none of these
 2H2X(g) + energy
2H2X(g) + energyThe equilibrium expression is
A)
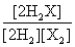
B)
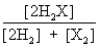
C)
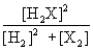
D)
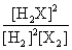
E) none of these

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
26
Consider the following equilibrium: 2H2(g) + X2(g)  2H2X(g) + energy
2H2X(g) + energy
Decreasing the volume of the container for this system at equilibrium will cause
A) an increase in the amount of H2X
B) an increase in the amounts of H2 and X2
C) an increase in the amount of H2 but not X2
D) no change
E) X2 to dissociate
 2H2X(g) + energy
2H2X(g) + energyDecreasing the volume of the container for this system at equilibrium will cause
A) an increase in the amount of H2X
B) an increase in the amounts of H2 and X2
C) an increase in the amount of H2 but not X2
D) no change
E) X2 to dissociate

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
27
Consider the reaction 2H2(g) + O2(g)  2H2O(g) at some equilibrium position. Using the following choices, indicate what will happen if the changes below are made.
2H2O(g) at some equilibrium position. Using the following choices, indicate what will happen if the changes below are made.
Some He(g) is injected into the reaction vessel.
A) shifts to the left
B) shifts to the right
C) no change
 2H2O(g) at some equilibrium position. Using the following choices, indicate what will happen if the changes below are made.
2H2O(g) at some equilibrium position. Using the following choices, indicate what will happen if the changes below are made.Some He(g) is injected into the reaction vessel.
A) shifts to the left
B) shifts to the right
C) no change

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
28
The concentrations of pure solids or pure liquids involved in a chemical reaction are not included in the equilibrium expression for the reaction.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
29
Given the equation A(g) ![<strong>Given the equation A(g) B(g) + 2C(g). At a particular temperature, K = 1.4 *10<sup>5</sup>. -Placing the equilibrium mixture in an ice bath (thus lowering the temperature)</strong> A) will cause [A] to increase B) will cause [B] to increase C) will have no effect D) cannot be determined E) none of the above](https://storage.examlex.com/TB6421/11eab077_f802_c82c_beed_d7913e44b56b_TB6421_11_TB6421_11_TB6421_11_TB6421_11.jpg) B(g) + 2C(g). At a particular temperature, K = 1.4 *105.
B(g) + 2C(g). At a particular temperature, K = 1.4 *105.
-Placing the equilibrium mixture in an ice bath (thus lowering the temperature)
A) will cause [A] to increase
B) will cause [B] to increase
C) will have no effect
D) cannot be determined
E) none of the above
![<strong>Given the equation A(g) B(g) + 2C(g). At a particular temperature, K = 1.4 *10<sup>5</sup>. -Placing the equilibrium mixture in an ice bath (thus lowering the temperature)</strong> A) will cause [A] to increase B) will cause [B] to increase C) will have no effect D) cannot be determined E) none of the above](https://storage.examlex.com/TB6421/11eab077_f802_c82c_beed_d7913e44b56b_TB6421_11_TB6421_11_TB6421_11_TB6421_11.jpg) B(g) + 2C(g). At a particular temperature, K = 1.4 *105.
B(g) + 2C(g). At a particular temperature, K = 1.4 *105.-Placing the equilibrium mixture in an ice bath (thus lowering the temperature)
A) will cause [A] to increase
B) will cause [B] to increase
C) will have no effect
D) cannot be determined
E) none of the above

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
30
Consider the reaction system CH4(g) + 2O2(g)  CO2(g) + 2H2O(g) + energy, and use the following choices to describe what happens when the changes below are made to the system at equilibrium.
CO2(g) + 2H2O(g) + energy, and use the following choices to describe what happens when the changes below are made to the system at equilibrium.
O2(g) is removed from the reaction vessel.
A) shifts to the left
B) shifts to the right
C) no change
 CO2(g) + 2H2O(g) + energy, and use the following choices to describe what happens when the changes below are made to the system at equilibrium.
CO2(g) + 2H2O(g) + energy, and use the following choices to describe what happens when the changes below are made to the system at equilibrium.O2(g) is removed from the reaction vessel.
A) shifts to the left
B) shifts to the right
C) no change

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
31
Consider the reaction system CH4(g) + 2O2(g)  CO2(g) + 2H2O(g) + energy, and use the following choices to describe what happens when the changes below are made to the system at equilibrium.
CO2(g) + 2H2O(g) + energy, and use the following choices to describe what happens when the changes below are made to the system at equilibrium.
He(g) is added to the reaction vessel.
A) shifts to the left
B) shifts to the right
C) no change
 CO2(g) + 2H2O(g) + energy, and use the following choices to describe what happens when the changes below are made to the system at equilibrium.
CO2(g) + 2H2O(g) + energy, and use the following choices to describe what happens when the changes below are made to the system at equilibrium.He(g) is added to the reaction vessel.
A) shifts to the left
B) shifts to the right
C) no change

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
32
Consider the reaction system CH4(g) + 2O2(g)  CO2(g) + 2H2O(g) + energy, and use the following choices to describe what happens when the changes below are made to the system at equilibrium.
CO2(g) + 2H2O(g) + energy, and use the following choices to describe what happens when the changes below are made to the system at equilibrium.
CH4(g) is added to the reaction vessel.
A) shifts to the left
B) shifts to the right
C) no change
 CO2(g) + 2H2O(g) + energy, and use the following choices to describe what happens when the changes below are made to the system at equilibrium.
CO2(g) + 2H2O(g) + energy, and use the following choices to describe what happens when the changes below are made to the system at equilibrium.CH4(g) is added to the reaction vessel.
A) shifts to the left
B) shifts to the right
C) no change

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
33
Consider the following equilibrium: 2H2(g) + X2(g) ![<strong>Consider the following equilibrium: 2H<sub>2</sub>(g) + X<sub>2</sub>(g) 2H<sub>2</sub>X(g) + energy Addition of argon to this system at equilibrium</strong> A) will cause [H<sub>2</sub>] to decrease B) will cause [X<sub>2</sub>] to increase C) will cause [H<sub>2</sub>X] to increase D) will have no effect E) cannot possibly be carried out](https://storage.examlex.com/TB6421/11eab077_f800_cc4d_beed_7b315b98091a_TB6421_11.jpg) 2H2X(g) + energy
2H2X(g) + energy
Addition of argon to this system at equilibrium
A) will cause [H2] to decrease
B) will cause [X2] to increase
C) will cause [H2X] to increase
D) will have no effect
E) cannot possibly be carried out
![<strong>Consider the following equilibrium: 2H<sub>2</sub>(g) + X<sub>2</sub>(g) 2H<sub>2</sub>X(g) + energy Addition of argon to this system at equilibrium</strong> A) will cause [H<sub>2</sub>] to decrease B) will cause [X<sub>2</sub>] to increase C) will cause [H<sub>2</sub>X] to increase D) will have no effect E) cannot possibly be carried out](https://storage.examlex.com/TB6421/11eab077_f800_cc4d_beed_7b315b98091a_TB6421_11.jpg) 2H2X(g) + energy
2H2X(g) + energyAddition of argon to this system at equilibrium
A) will cause [H2] to decrease
B) will cause [X2] to increase
C) will cause [H2X] to increase
D) will have no effect
E) cannot possibly be carried out

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
34
Given the equation A(g) ![<strong>Given the equation A(g) B(g) + 2C(g). At a particular temperature, K = 1.4 *10<sup>5</sup>. -Raising the pressure by decreasing the volume of the container</strong> A) will cause [A] to increase B) will cause [B] to increase C) will have no effect D) cannot be determined E) none of the above](https://storage.examlex.com/TB6421/11eab077_f802_c82c_beed_d7913e44b56b_TB6421_11_TB6421_11_TB6421_11_TB6421_11.jpg) B(g) + 2C(g). At a particular temperature, K = 1.4 *105.
B(g) + 2C(g). At a particular temperature, K = 1.4 *105.
-Raising the pressure by decreasing the volume of the container
A) will cause [A] to increase
B) will cause [B] to increase
C) will have no effect
D) cannot be determined
E) none of the above
![<strong>Given the equation A(g) B(g) + 2C(g). At a particular temperature, K = 1.4 *10<sup>5</sup>. -Raising the pressure by decreasing the volume of the container</strong> A) will cause [A] to increase B) will cause [B] to increase C) will have no effect D) cannot be determined E) none of the above](https://storage.examlex.com/TB6421/11eab077_f802_c82c_beed_d7913e44b56b_TB6421_11_TB6421_11_TB6421_11_TB6421_11.jpg) B(g) + 2C(g). At a particular temperature, K = 1.4 *105.
B(g) + 2C(g). At a particular temperature, K = 1.4 *105.-Raising the pressure by decreasing the volume of the container
A) will cause [A] to increase
B) will cause [B] to increase
C) will have no effect
D) cannot be determined
E) none of the above

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
35
Consider the reaction 2H2(g) + O2(g)  2H2O(g) at some equilibrium position. Using the following choices, indicate what will happen if the changes below are made.
2H2O(g) at some equilibrium position. Using the following choices, indicate what will happen if the changes below are made.
The size of the reaction vessel is decreased.
A) shifts to the left
B) shifts to the right
C) no change
 2H2O(g) at some equilibrium position. Using the following choices, indicate what will happen if the changes below are made.
2H2O(g) at some equilibrium position. Using the following choices, indicate what will happen if the changes below are made.The size of the reaction vessel is decreased.
A) shifts to the left
B) shifts to the right
C) no change

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
36
In the presence of ultraviolet light, the "inert" gas xenon (Xe) will react with fluorine (F2) gas to produce solid XeF4. What is the equilibrium expression for this reaction?
A)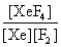
B)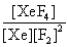
C)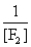
D)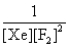
E)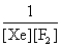
A)
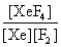
B)
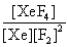
C)
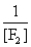
D)
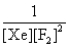
E)
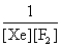

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
37
Consider the reaction system CH4(g) + 2O2(g)  CO2(g) + 2H2O(g) + energy, and use the following choices to describe what happens when the changes below are made to the system at equilibrium.
CO2(g) + 2H2O(g) + energy, and use the following choices to describe what happens when the changes below are made to the system at equilibrium.
CO2(g) is removed from the reaction vessel.
A) shifts to the left
B) shifts to the right
C) no change
 CO2(g) + 2H2O(g) + energy, and use the following choices to describe what happens when the changes below are made to the system at equilibrium.
CO2(g) + 2H2O(g) + energy, and use the following choices to describe what happens when the changes below are made to the system at equilibrium.CO2(g) is removed from the reaction vessel.
A) shifts to the left
B) shifts to the right
C) no change

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
38
Consider a system of four gases. The equilibrium concentration of each product is 2.8 M. The equilibrium concentrations of the reactants are equal. The equilibrium is shown here: A + B  C + D K = 2.6
C + D K = 2.6
What is the equilibrium concentration of gas A?
A) 3.0 M
B) 20.4 M
C) 7.3 M
D) 1.7 M
E) 0.58 M
 C + D K = 2.6
C + D K = 2.6What is the equilibrium concentration of gas A?
A) 3.0 M
B) 20.4 M
C) 7.3 M
D) 1.7 M
E) 0.58 M

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
39
Consider the reaction system CH4(g) + 2O2(g)  CO2(g) + 2H2O(g) + energy, and use the following choices to describe what happens when the changes below are made to the system at equilibrium.
CO2(g) + 2H2O(g) + energy, and use the following choices to describe what happens when the changes below are made to the system at equilibrium.
The temperature is increased.
A) shifts to the left
B) shifts to the right
C) no change
 CO2(g) + 2H2O(g) + energy, and use the following choices to describe what happens when the changes below are made to the system at equilibrium.
CO2(g) + 2H2O(g) + energy, and use the following choices to describe what happens when the changes below are made to the system at equilibrium.The temperature is increased.
A) shifts to the left
B) shifts to the right
C) no change

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
40
Given the equation A(g) ![<strong>Given the equation A(g) B(g) + 2C(g). At a particular temperature, K = 1.4 *10<sup>5</sup>. -Addition of chemical B to an equilibrium mixture of the above</strong> A) will cause [A] to increase B) will cause [C] to increase C) will have no effect D) cannot be determined E) none of the above](https://storage.examlex.com/TB6421/11eab077_f802_c82c_beed_d7913e44b56b_TB6421_11_TB6421_11_TB6421_11_TB6421_11.jpg) B(g) + 2C(g). At a particular temperature, K = 1.4 *105.
B(g) + 2C(g). At a particular temperature, K = 1.4 *105.
-Addition of chemical B to an equilibrium mixture of the above
A) will cause [A] to increase
B) will cause [C] to increase
C) will have no effect
D) cannot be determined
E) none of the above
![<strong>Given the equation A(g) B(g) + 2C(g). At a particular temperature, K = 1.4 *10<sup>5</sup>. -Addition of chemical B to an equilibrium mixture of the above</strong> A) will cause [A] to increase B) will cause [C] to increase C) will have no effect D) cannot be determined E) none of the above](https://storage.examlex.com/TB6421/11eab077_f802_c82c_beed_d7913e44b56b_TB6421_11_TB6421_11_TB6421_11_TB6421_11.jpg) B(g) + 2C(g). At a particular temperature, K = 1.4 *105.
B(g) + 2C(g). At a particular temperature, K = 1.4 *105.-Addition of chemical B to an equilibrium mixture of the above
A) will cause [A] to increase
B) will cause [C] to increase
C) will have no effect
D) cannot be determined
E) none of the above

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
41
The solubility of BaCO3(s) in water at a certain temperature is 4.4*10-5 mol/L. Calculate the value of Ksp for BaCO3(s) at this temperature.
A) 4.4 * 10-5
B) 8.8 * 10-5
C) 1.9* 10-9
D) 6.6 * 10-3
E) none of these
A) 4.4 * 10-5
B) 8.8 * 10-5
C) 1.9* 10-9
D) 6.6 * 10-3
E) none of these

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
42
Calculate the concentration of the copper ion is a saturated solution of copper(I) cyanide, CuCN (Ksp = 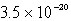 ).
).
A)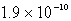
B)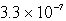
C)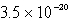
D)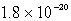
E) none of these
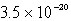 ).
).A)
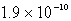
B)
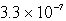
C)
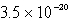
D)
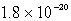
E) none of these

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
43
The solubility of ZnS(s) in water at a certain temperature is 1.7 * 10-11 mol/L. The value of the Ksp of ZnS is
A) 2.9 * 10-22
B) 1.7 * 10-11
C) 4.1 * 10-6
D) 8.5 *10-12
E) 3.4 * 10-11
A) 2.9 * 10-22
B) 1.7 * 10-11
C) 4.1 * 10-6
D) 8.5 *10-12
E) 3.4 * 10-11

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
44
Write the balanced equation for the dissolving of Ag2S(s) in water.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
45
Write the balanced equation for the dissolving of Fe3(PO4)2 in water.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
46
Lithium carbonate, Li2CO3 has a Ksp of 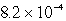 at a certain temperature. What is the solubility in mol/L for Li2CO3 at this temperature?
at a certain temperature. What is the solubility in mol/L for Li2CO3 at this temperature?
A)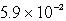
B)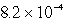
C)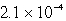
D)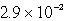
E)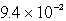
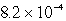 at a certain temperature. What is the solubility in mol/L for Li2CO3 at this temperature?
at a certain temperature. What is the solubility in mol/L for Li2CO3 at this temperature?A)
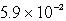
B)
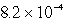
C)
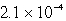
D)
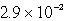
E)
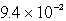

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
47
Write the balanced equation for the dissolving of PbCl2(s) in water.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
48
Given the solubility products (Ksp) BaSO4
1.5* 10-9
CoS
5.0 * 10-22
PbSO4
1.3 *10-2
AgBr
5.0 * 10-13
Which of the following compounds is the most soluble (in mol/L)?
A) BaSO4
B) CoS
C) PbSO4
D) AgBr
E) BaCO3
1.5* 10-9
CoS
5.0 * 10-22
PbSO4
1.3 *10-2
AgBr
5.0 * 10-13
Which of the following compounds is the most soluble (in mol/L)?
A) BaSO4
B) CoS
C) PbSO4
D) AgBr
E) BaCO3

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
49
The Ksp for ZnS(s) is 3.0 *10-22 at a certain temperature. The solubility of ZnS(s) in water at this temperature is
A)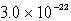
B)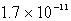
C)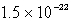
D)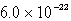
E) none of these
A)
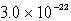
B)
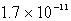
C)
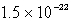
D)
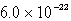
E) none of these

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
50
The solubility in mol/L of Ag2CrO4 is 1.3 F* 10-4 M at a certain temperature. What is the Ksp for this compound?
A) 1.3 * 10-4
B) 2.2 *10-12
C) 8.8 *10-12
D) 3.4 *10-8
E) 6.8*10-8
A) 1.3 * 10-4
B) 2.2 *10-12
C) 8.8 *10-12
D) 3.4 *10-8
E) 6.8*10-8

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
51
The solubility of Mg(OH)2(s) in water at a certain temperature is 1.1 *10-4 mol/L. Calculate the value of Ksp for Mg(OH)2(s) at this temperature.
A)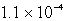
B)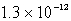
C)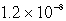
D)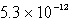
E) none of these
A)
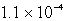
B)
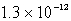
C)
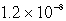
D)
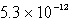
E) none of these

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
52
For the reaction 2SO2(g) + O2(g) ![<strong>For the reaction 2SO<sub>2</sub>(g) + O<sub>2</sub>(g) 2SO<sub>3</sub>(g) At a certain temperature, the equilibrium concentrations were observed to be [SO<sub>2</sub>] = 0.564 M, [O<sub>2</sub>] = 7.50*10<sup>-</sup><sup>2</sup> M, and [SO<sub>3</sub>] = 0.650 M. Calculate the value of K for this system at this temperature.</strong> A) 15.4 B) 27.2 C) 9.99 D) 236 E) 17.7](https://storage.examlex.com/TB6421/11eab077_f803_8b7e_beed_9f441490cd04_TB6421_11.jpg) 2SO3(g)
2SO3(g)
At a certain temperature, the equilibrium concentrations were observed to be [SO2] = 0.564 M, [O2] = 7.50*10-2 M, and [SO3] = 0.650 M. Calculate the value of K for this system at this temperature.
A) 15.4
B) 27.2
C) 9.99
D) 236
E) 17.7
![<strong>For the reaction 2SO<sub>2</sub>(g) + O<sub>2</sub>(g) 2SO<sub>3</sub>(g) At a certain temperature, the equilibrium concentrations were observed to be [SO<sub>2</sub>] = 0.564 M, [O<sub>2</sub>] = 7.50*10<sup>-</sup><sup>2</sup> M, and [SO<sub>3</sub>] = 0.650 M. Calculate the value of K for this system at this temperature.</strong> A) 15.4 B) 27.2 C) 9.99 D) 236 E) 17.7](https://storage.examlex.com/TB6421/11eab077_f803_8b7e_beed_9f441490cd04_TB6421_11.jpg) 2SO3(g)
2SO3(g)At a certain temperature, the equilibrium concentrations were observed to be [SO2] = 0.564 M, [O2] = 7.50*10-2 M, and [SO3] = 0.650 M. Calculate the value of K for this system at this temperature.
A) 15.4
B) 27.2
C) 9.99
D) 236
E) 17.7

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
53
The solubility of Co(OH)2 in water at a certain temperature is 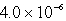 mol/L. The value of Ksp of Co(OH)2 at this temperature is
mol/L. The value of Ksp of Co(OH)2 at this temperature is
A)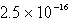
B)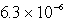
C)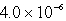
D)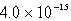
E) none of these
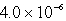 mol/L. The value of Ksp of Co(OH)2 at this temperature is
mol/L. The value of Ksp of Co(OH)2 at this temperature isA)
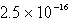
B)
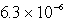
C)
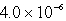
D)
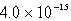
E) none of these

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
54
For the reaction CO(g) + H2O(g) ![<strong>For the reaction CO(g) + H<sub>2</sub>O(g) CO<sub>2</sub>(g) + H<sub>2</sub>(g) K = 3.88 at a certain temperature. If at this temperature in a certain experiment the equilibrium concentrations are [H<sub>2</sub>] = 1.5 M, [CO<sub>2</sub>] = 1.8 M, and [H<sub>2</sub>O] = 0.26 M, calculate [CO].</strong> A) 0.37 M B) 1.5 M C) 4.7 M D) 2.7 M E) none of these](https://storage.examlex.com/TB6421/11eab077_f803_d99f_beed_65f1b8fe7307_TB6421_11.jpg) CO2(g) + H2(g)
CO2(g) + H2(g)
K = 3.88 at a certain temperature. If at this temperature in a certain experiment the equilibrium concentrations are [H2] = 1.5 M, [CO2] = 1.8 M, and [H2O] = 0.26 M, calculate [CO].
A) 0.37 M
B) 1.5 M
C) 4.7 M
D) 2.7 M
E) none of these
![<strong>For the reaction CO(g) + H<sub>2</sub>O(g) CO<sub>2</sub>(g) + H<sub>2</sub>(g) K = 3.88 at a certain temperature. If at this temperature in a certain experiment the equilibrium concentrations are [H<sub>2</sub>] = 1.5 M, [CO<sub>2</sub>] = 1.8 M, and [H<sub>2</sub>O] = 0.26 M, calculate [CO].</strong> A) 0.37 M B) 1.5 M C) 4.7 M D) 2.7 M E) none of these](https://storage.examlex.com/TB6421/11eab077_f803_d99f_beed_65f1b8fe7307_TB6421_11.jpg) CO2(g) + H2(g)
CO2(g) + H2(g)K = 3.88 at a certain temperature. If at this temperature in a certain experiment the equilibrium concentrations are [H2] = 1.5 M, [CO2] = 1.8 M, and [H2O] = 0.26 M, calculate [CO].
A) 0.37 M
B) 1.5 M
C) 4.7 M
D) 2.7 M
E) none of these

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
55
The solubility of Cd(OH)2 in water at a certain temperature is 1.9 *10-5 mol/L. The Ksp value for Cd(OH)2 is
A) 2.7 * 10-14
B) 1.9 *10-5
C) 6.9 * 10-15
D) 7.2* 10-10
E) 3.6 * 10-10
A) 2.7 * 10-14
B) 1.9 *10-5
C) 6.9 * 10-15
D) 7.2* 10-10
E) 3.6 * 10-10

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
56
The molar solubility of PbI2 at a certain temperature is 1.7 *10-3 M. Calculate the value of Ksp for PbI2.
A)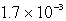
B)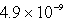
C)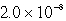
D)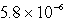
E) none of these
A)
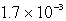
B)
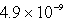
C)
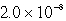
D)
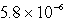
E) none of these

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck


