Deck 6: Chemical Reactions: an Introduction
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/76
Play
Full screen (f)
Deck 6: Chemical Reactions: an Introduction
1
You are asked to balance the chemical equation H2 + O2 H2O. How many of the following ways are correct ways to balance this equation? I. 2H2 + O2 2H2O
II) H2 +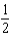 O2 H2O
O2 H2O
III) 4H2 + 2O2 4H2O
IV) H2 + O2 H2O2
A) 0
B) 1
C) 2
D) 3
E) 4
II) H2 +
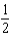 O2 H2O
O2 H2OIII) 4H2 + 2O2 4H2O
IV) H2 + O2 H2O2
A) 0
B) 1
C) 2
D) 3
E) 4
3
2
When the following equation is balanced using the smallest possible integers, what is the number in front of the substance in bold type? PCl5 + H2O H3PO4 + HCl
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5
5
3
When the following equation is balanced using the smallest possible integers, what is the number in front of the substance in bold type? Al + Fe3O4 Al2O3 + Fe
A) 1
B) 3
C) 6
D) 9
E) 12
A) 1
B) 3
C) 6
D) 9
E) 12
9
4
All of the following are clues that a chemical reaction has taken place except
A) A color change occurs.
B) A solid forms.
C) The reactant is smaller.
D) Bubbles form.
E) A flame occurs.
A) A color change occurs.
B) A solid forms.
C) The reactant is smaller.
D) Bubbles form.
E) A flame occurs.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
5
When the following equation is balanced using the smallest possible integers, what is the number in front of the substance in bold type? NO2 + H2O HNO3 + NO
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
6
When the following equation is balanced using the smallest possible integers, what is the number in front of the substance in bold type? CH3OH + O2 CO2 + H2O
A) 1
B) 2
C) 3
D) 4
E) 6
A) 1
B) 2
C) 3
D) 4
E) 6

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
7
The equation N2 + 3H2 2NH3 means that 1 g of N2 reacts with 3 g of H2 to form 2 g of NH3.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
8
Consider the reaction represented by the unbalanced equation NH3 + O2 NO + H2O. For every 3.61 mol of NH3 that reacts, _______ mol of O2 is required.
A) 4.51
B) 3.61
C) 14.44
D) 2.89
E) none of these
A) 4.51
B) 3.61
C) 14.44
D) 2.89
E) none of these

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
9
When the following equation is balanced using the smallest possible integers, what is the number in front of the substance in bold type? Pb(NO3)2 + K2CO3 PbCO3 + KNO3
A) 5
B) 4
C) 3
D) 2
E) 1
A) 5
B) 4
C) 3
D) 2
E) 1

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
10
Sodium metal reacts with water to produce aqueous sodium hydroxide and hydrogen gas. Write the balanced equation for this reaction.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
11
When the following equation is balanced using the smallest possible integers, what is the number in front of the substance in bold type? Na2S2O3 + I2 NaI + Na2S4O6
A) 1
B) 2
C) 3
D) 4
E) 6
A) 1
B) 2
C) 3
D) 4
E) 6

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
12
Coefficients can be fractions when we are balancing a chemical equation.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
13
Balance the following equation in standard form and determine the sum of the coefficients. 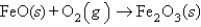
A) 3
B) 4
C) 6
D) 7
E) 14
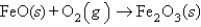
A) 3
B) 4
C) 6
D) 7
E) 14

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
14
Consider a reaction given by the equation aA + bB cC + dD. In this equation A, B, C, D represent chemicals, and a, b, c, d represent coefficients in the balanced equation. For a given reaction, how many values are there for the quantity "c/d"?
A) 1
B) 2
C) 3
D) 4
E) an infinite number
A) 1
B) 2
C) 3
D) 4
E) an infinite number

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
15
Balance the following equation in standard form and determine the sum of the coefficients. LiAlH4(s) + AlCl3(s) AlH3(s) + LiCl(s)
A) 8
B) 9
C) 10
D) 11
E) 12
A) 8
B) 9
C) 10
D) 11
E) 12

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
16
When the following equation is balanced using the smallest possible integers, what is the number in front of the substance in bold type? P4O10 + H2O H3PO4
A) 10
B) 6
C) 4
D) 2
E) 1
A) 10
B) 6
C) 4
D) 2
E) 1

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following statements is not true of balancing a chemical equation?
A) Subscripts in the reactants must be conserved in the products.
B) Coefficients are used to balance the atoms on both sides.
C) The law of conservation of matter must be followed.
D) Phases are often shown for each compound but are not critical to balancing an equation.
E) All of the above statements (a-d) are true.
A) Subscripts in the reactants must be conserved in the products.
B) Coefficients are used to balance the atoms on both sides.
C) The law of conservation of matter must be followed.
D) Phases are often shown for each compound but are not critical to balancing an equation.
E) All of the above statements (a-d) are true.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
18
When the following equation is balanced using the smallest possible integers, what is the number in front of the substance in bold type? Sn + NaOH Na2SnO2 + H2
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
19
Aluminum oxide solid reacts with gaseous carbon monoxide to produce aluminum metal and carbon dioxide gas. Write the balanced equation for this reaction.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
20
When the following equation is balanced using the smallest possible integers, what is the number in front of the substance in bold type? MnO2 + HCl MnCl2 + Cl2 + H2O
A) 5
B) 4
C) 3
D) 2
E) 1
A) 5
B) 4
C) 3
D) 2
E) 1

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
21
Balance the equation
MgCl2 + K3PO4 Mg3(PO4)2 + KCl
MgCl2 + K3PO4 Mg3(PO4)2 + KCl

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
22
Balance the equation
Sb(s) + O2(g) Sb2O3(s)
Sb(s) + O2(g) Sb2O3(s)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
23
Balance the equation
NaBH4 + BF3 NaBF4 + B2H6
NaBH4 + BF3 NaBF4 + B2H6

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
24
When table sugar is burned in air, carbon dioxide and water vapor are products, as shown by the following unbalanced chemical equation: C12H22O11(s) + O2(g) CO2(g) + H2O(g)
How many moles of oxygen are required to react completely with 1.26 mol of sugar?
A) 15.1
B) 1.26
C) 22
D) 30
E) none of these
How many moles of oxygen are required to react completely with 1.26 mol of sugar?
A) 15.1
B) 1.26
C) 22
D) 30
E) none of these

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
25
When the following equation is balanced using the smallest possible integers, what is the number in front of the substance in bold type? NBr3 + NaOH N2 + NaBr + HOBr
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
26
Balance the equation
KClO3(s) KCl(s) + O2(g)
KClO3(s) KCl(s) + O2(g)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
27
When the following equations are balanced using the smallest possible integers, what is the number in front of the underlined substance in each case?
-S(s) + O2(g) SO2(g)
A) 1
B) 2
C) 3
D) 4
E) 5
-S(s) + O2(g) SO2(g)
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
28
Balance the equation
As2O3(s) + Ca(OH)2(aq) Ca3(AsO4)2(s) + H2O(l)
As2O3(s) + Ca(OH)2(aq) Ca3(AsO4)2(s) + H2O(l)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
29
When the following equations are balanced using the smallest possible integers, what is the number in front of the underlined substance in each case?
-Li(s) + O2(g) Li2O(s)
A) 1
B) 2
C) 3
D) 4
E) 5
-Li(s) + O2(g) Li2O(s)
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
30
When the following equations are balanced using the smallest possible integers, what is the number in front of the underlined substance in each case?
-Al2O3(s) + H2SO4(aq) Al2(SO4)3(aq) + H2O(l)
A) 1
B) 2
C) 3
D) 6
E) 9
-Al2O3(s) + H2SO4(aq) Al2(SO4)3(aq) + H2O(l)
A) 1
B) 2
C) 3
D) 6
E) 9

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
31
When ethane (C2H6) is reacted with oxygen in the air, the products are carbon dioxide and water. This process requires __________ mol of oxygen for every 2.43 mol of ethane.
A) 8.51
B) 6.08
C) 2.43
D) 17.01
E) none of these
A) 8.51
B) 6.08
C) 2.43
D) 17.01
E) none of these

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
32
When the following equation is balanced using the smallest possible integers, what is the number in front of the substance in bold type? KClO3 KCl + KClO4
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
33
When the following equations are balanced using the smallest possible integers, what is the number in front of the underlined substance in each case?
-Pb(s) + AgNO3(aq) Pb(NO3)2 + Ag(s)
A) 1
B) 2
C) 4
D) 5
E) 6
-Pb(s) + AgNO3(aq) Pb(NO3)2 + Ag(s)
A) 1
B) 2
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
34
When the following equation is balanced using the smallest possible integers, what is the number in front of the substance in bold type? C2H6 + O2 CO2 + H2O
A) 1
B) 2
C) 4
D) 6
E) 7
A) 1
B) 2
C) 4
D) 6
E) 7

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
35
Balance the equation
C6H14 + O2 CO2 + H2O
C6H14 + O2 CO2 + H2O

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
36
When the following equations are balanced using the smallest possible integers, what is the number in front of the underlined substance in each case?
-N2(g) + O2(g) N2O3
A) 1
B) 2
C) 3
D) 4
E) 6
-N2(g) + O2(g) N2O3
A) 1
B) 2
C) 3
D) 4
E) 6

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
37
When the following equations are balanced using the smallest possible integers, what is the number in front of the underlined substance in each case?
-C2H6(g) + O2(g) CO2(g) + H2O(g)
A) 4
B) 7
C) 8
D) 10
E) 14
-C2H6(g) + O2(g) CO2(g) + H2O(g)
A) 4
B) 7
C) 8
D) 10
E) 14

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
38
When the following equations are balanced using the smallest possible integers, what is the number in front of the underlined substance in each case?
-Sr(s) + P4(s) Sr3P2(s)
A) 2
B) 3
C) 6
D) 12
E) 18
-Sr(s) + P4(s) Sr3P2(s)
A) 2
B) 3
C) 6
D) 12
E) 18

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
39
When the following equation is balanced using the smallest possible integers, what is the number in front of the substance in bold type? SiCl4 + H2O SiO2 + HCl
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
40
Balance the equation
Mg(OH)2(aq) + 2HBr(aq) MgBr2(aq) + 2H2O(l)
Mg(OH)2(aq) + 2HBr(aq) MgBr2(aq) + 2H2O(l)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
41
Balance the equation
KI(aq) + Cl2(g) KCl(aq) + I2(s)
KI(aq) + Cl2(g) KCl(aq) + I2(s)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
42
Balance the equation for the reaction of aluminum metal with solid iodine to form solid aluminum iodide.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
43
Write and balance the equation showing the reaction between iron(III) oxide and carbon monoxide to form iron metal and carbon dioxide.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
44
Balance the equation for the reaction of calcium metal with oxygen gas to produce solid calcium oxide.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
45
Balance the equation
Pb(NO3)2(aq) + K2CrO4(aq) PbCrO4(s) + KNO3(aq)
Pb(NO3)2(aq) + K2CrO4(aq) PbCrO4(s) + KNO3(aq)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
46
Balance the equation
Mg(s) + O2(g) MgO(s)
Mg(s) + O2(g) MgO(s)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
47
Balance the equation for the reaction of potassium metal with water to form potassium hydroxide and hydrogen gas.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
48
When the following equations are balanced using the smallest possible integers, what is the number in front of the underlined substance in each case?
-C4H10(g) + O2(g) CO2(g) + H2O(g)
A) 2
B) 4
C) 6
D) 8
E) 10
-C4H10(g) + O2(g) CO2(g) + H2O(g)
A) 2
B) 4
C) 6
D) 8
E) 10

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
49
Balance the equation
H2O2(l) H2O(l) + O2(g)
H2O2(l) H2O(l) + O2(g)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
50
Balance the equation
(NH4)2Cr2O7(s) N2(g) + H2O(g) + Cr2O3(s)
(NH4)2Cr2O7(s) N2(g) + H2O(g) + Cr2O3(s)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
51
Write and balance the equation showing the reaction between iron(III) oxide and carbon monoxide to form iron(II) oxide and carbon dioxide.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
52
Balance the equation
BaCl2(aq) + H2SO4(aq) BaSO4(s) + HCl(aq)
BaCl2(aq) + H2SO4(aq) BaSO4(s) + HCl(aq)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
53
Write and balance the equation showing the reaction between gaseous hydrogen sulfide and oxygen to form sulfur dioxide gas and water vapor.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following statements about chemical reactions is false?
A) In balancing a chemical equation, all subscripts must be conserved.
B) When one coefficient is doubled, the rest of the coefficients in the balanced equation must also be doubled.
C) The subscripts in a balanced equation tell us the number of atoms in a molecule.
D) An individual coefficient in a balanced equation is meaningless.
E) The phases in a chemical reaction tell us the nature of the reactants and products.
A) In balancing a chemical equation, all subscripts must be conserved.
B) When one coefficient is doubled, the rest of the coefficients in the balanced equation must also be doubled.
C) The subscripts in a balanced equation tell us the number of atoms in a molecule.
D) An individual coefficient in a balanced equation is meaningless.
E) The phases in a chemical reaction tell us the nature of the reactants and products.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
55
Write and balance the equation showing the reaction between nitrogen monoxide gas and hydrogen gas to form nitrogen gas and water vapor.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
56
Write and balance the equation showing the reaction between calcium metal and water to form aqueous calcium hydroxide and hydrogen gas.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
57
Write and balance the equation showing the reaction between copper metal and aqueous sulfuric acid to form aqueous copper(II) sulfate, sulfur dioxide gas, and water.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
58
When the following equations are balanced using the smallest possible integers, what is the number in front of the underlined substance in each case?
-Sb(s) + O2(g) Sb2O5(s)
A) 1
B) 2
C) 4
D) 6
E) 12
-Sb(s) + O2(g) Sb2O5(s)
A) 1
B) 2
C) 4
D) 6
E) 12

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
59
Balance the equation
Zn(s) + HCl(aq) H2(g) + ZnCl2(aq)
Zn(s) + HCl(aq) H2(g) + ZnCl2(aq)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
60
When the following equations are balanced using the smallest possible integers, what is the number in front of the underlined substance in each case?
-CH3OH(l) + O2(g) CO2(g) + H2O(g)
A) 1
B) 2
C) 4
D) 6
E) 12
-CH3OH(l) + O2(g) CO2(g) + H2O(g)
A) 1
B) 2
C) 4
D) 6
E) 12

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
61
Balance the equation
Al(s) + S(s) Al2S3(s)
Al(s) + S(s) Al2S3(s)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
62
Balance the equation
C8H18(l) + O2(g) CO2(g) + H2O(g)
C8H18(l) + O2(g) CO2(g) + H2O(g)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
63
Balance the equation
CaCO3(s) + HCl(aq) H2O(l) + CO2(g) + CaCl2(aq)
CaCO3(s) + HCl(aq) H2O(l) + CO2(g) + CaCl2(aq)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
64
Balance the equation
K(s) + H2O(l) KOH(aq) + H2(g)
K(s) + H2O(l) KOH(aq) + H2(g)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
65
Balance the equation
HCl(aq) + Ca(OH)2(aq) CaCl2(aq) + H2O(l)
HCl(aq) + Ca(OH)2(aq) CaCl2(aq) + H2O(l)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
66
Balance the equation
Ca(s) + O2(g) CaO(s)
Ca(s) + O2(g) CaO(s)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
67
When the equation Si(s) + HF(aq) SiF4(g) + H2(g) is balanced, what is the coefficient for HF?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
68
Balance the equation
Mg3N2(s) + H2O(l) NH3(g) + Mg(OH)2(s)
Mg3N2(s) + H2O(l) NH3(g) + Mg(OH)2(s)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
69
Balance the equation
Zn(s) + H3PO4(aq) Zn3(PO4)2(s) + H2(g)
Zn(s) + H3PO4(aq) Zn3(PO4)2(s) + H2(g)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
70
Balance the equation
Na2O(s) + H2O(l) NaOH(aq)
Na2O(s) + H2O(l) NaOH(aq)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
71
Balance the equation
C3H7OH(l) + O2(g) CO2(g) + H2O(g)
C3H7OH(l) + O2(g) CO2(g) + H2O(g)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
72
Balance the equation
Fe(NO3)2(aq) + H2S(g) FeS(s) + HNO3(aq)
Fe(NO3)2(aq) + H2S(g) FeS(s) + HNO3(aq)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
73
Balance the equation
Na(s) + H2O(l) H2(g) + NaOH(aq)
Na(s) + H2O(l) H2(g) + NaOH(aq)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
74
Balance the equation
AgNO3(aq) + CaCl2(aq) AgCl(s) + Ca(NO3)2(aq)
AgNO3(aq) + CaCl2(aq) AgCl(s) + Ca(NO3)2(aq)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
75
Balance the equation
PBr3(g) + H2O(l) H3PO3(aq) + HBr(aq)
PBr3(g) + H2O(l) H3PO3(aq) + HBr(aq)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
76
Balance the equation
NH3(g) + O2(g) NO(g) + H2O(l)
NH3(g) + O2(g) NO(g) + H2O(l)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck


