Deck 4: Chemical Foundations: Elements, Atoms, and Ions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/101
Play
Full screen (f)
Deck 4: Chemical Foundations: Elements, Atoms, and Ions
1
The symbol Ga stands for the element
A) gallium
B) germanium
C) gold
D) gadolinium
E) none of these
A) gallium
B) germanium
C) gold
D) gadolinium
E) none of these
gallium
2
The symbol for the element boron is
A) B
B) Bn
C) Be
D) Bor
E) Bm
A) B
B) Bn
C) Be
D) Bor
E) Bm
B
3
What is the most abundant element in the human body?
A) carbon
B) hydrogen
C) calcium
D) oxygen
E) water
A) carbon
B) hydrogen
C) calcium
D) oxygen
E) water
oxygen
4
The symbol P stands for the element
A) platinum
B) palladium
C) plutonium
D) potassium
E) phosphorus
A) platinum
B) palladium
C) plutonium
D) potassium
E) phosphorus

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
5
The symbol Cd stands for the element
A) cadmium
B) candelium
C) calcium
D) cesium
E) carbon
A) cadmium
B) candelium
C) calcium
D) cesium
E) carbon

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
6
What is the most abundant element on the earth (including the crust, oceans, and atmosphere)?
A) silicon
B) oxygen
C) hydrogen
D) carbon
E) iron
A) silicon
B) oxygen
C) hydrogen
D) carbon
E) iron

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
7
The symbol for the element titanium is
A) T
B) Tt
C) Ti
D) Tm
E) Tn
A) T
B) Tt
C) Ti
D) Tm
E) Tn

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
8
Four of the most important elements on this planet are hydrogen, oxygen, carbon, and nitrogen. Give the symbol for each of these elements.
a. hydrogen ______________
b. oxygen ______________
c. carbon ______________
d. nitrogen ______________
a. hydrogen ______________
b. oxygen ______________
c. carbon ______________
d. nitrogen ______________

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
9
The symbol for the element zinc is
A) Zn
B) Z
C) Zi
D) Zc
E) Zin
A) Zn
B) Z
C) Zi
D) Zc
E) Zin

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
10
Write the name for Kr. ______________

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
11
The symbol N stands for the element
A) nitrogen
B) nickel
C) neon
D) nobelium
E) niobium
A) nitrogen
B) nickel
C) neon
D) nobelium
E) niobium

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
12
The symbol Fe stands for the element
A) francium
B) fluorine
C) fermium
D) tin
E) iron
A) francium
B) fluorine
C) fermium
D) tin
E) iron

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
13
The symbol for the element strontium is
A) S
B) St
C) Sm
D) Str
E) Sr
A) S
B) St
C) Sm
D) Str
E) Sr

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
14
Write the name for Zr. ______________

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
15
Write the name for K. ______________

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
16
The symbol for aluminum is
A) A
B) Am
C) Al
D) Au
E) An
A) A
B) Am
C) Al
D) Au
E) An

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
17
The symbol for the element cesium is
A) Cs
B) Cm
C) Ca
D) Cr
E) Cu
A) Cs
B) Cm
C) Ca
D) Cr
E) Cu

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
18
The symbol for lead is
A) Ld
B) Pb
C) P
D) L
E) Le
A) Ld
B) Pb
C) P
D) L
E) Le

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
19
The symbol for the element iodine is
A) Io
B) In
C) Id
D) I
E) Ie
A) Io
B) In
C) Id
D) I
E) Ie

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
20
The symbol Sn stands for the element
A) sulfur
B) strontium
C) silicon
D) tin
E) sodium
A) sulfur
B) strontium
C) silicon
D) tin
E) sodium

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following statements are true? I. Models are always wrong unless they are proved by a theory.
II) Elements, such as lead, are made of tiny particles that mostly consist of open space.
III) The air you breathe is an example of a heterogeneous mixture.
IV) Because NH3 always contains the same relative numbers of atoms, it will always contain 4.6 g of nitrogen for every 1.0 g of hydrogen.
A) II only
B) II, IV
C) I, II, IV
D) I, III
E) All of the above statements are true.
II) Elements, such as lead, are made of tiny particles that mostly consist of open space.
III) The air you breathe is an example of a heterogeneous mixture.
IV) Because NH3 always contains the same relative numbers of atoms, it will always contain 4.6 g of nitrogen for every 1.0 g of hydrogen.
A) II only
B) II, IV
C) I, II, IV
D) I, III
E) All of the above statements are true.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
22
Write the symbol for manganese. ______________

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
23
Which statement below is incorrect?
A) Dalton's statement "All atoms of a given element are identical" is no longer accepted because of the existence of isotopes and ions.
B) O3 (ozone) is considered a molecule but is not considered an element.
C) One mole of aluminum is equal to the number of aluminum atoms in 26.98 g of aluminum.
D) Gases behave the most ideally at high temperatures and low pressures.
E) At least two of the above statements (a-d) are incorrect.
A) Dalton's statement "All atoms of a given element are identical" is no longer accepted because of the existence of isotopes and ions.
B) O3 (ozone) is considered a molecule but is not considered an element.
C) One mole of aluminum is equal to the number of aluminum atoms in 26.98 g of aluminum.
D) Gases behave the most ideally at high temperatures and low pressures.
E) At least two of the above statements (a-d) are incorrect.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
24
How many atoms of hydrogen are in five molecules of C5H6Cl?
A) 30
B) 5 x 1023
C) 6
D) 10 x 1023
E) 6 x 1023
A) 30
B) 5 x 1023
C) 6
D) 10 x 1023
E) 6 x 1023

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following does Dalton's Atomic Theory not address?
A) Hydrogen and oxygen gas combine to form water.
B) H2O2 consists of 16 g of oxygen for every 1 g of hydrogen.
C) The seat you are sitting on is made up of atoms.
D) Hydrogen ignites in an open flame but helium does not.
E) The relative mass of a carbon atom is about 12 times that of hydrogen.
A) Hydrogen and oxygen gas combine to form water.
B) H2O2 consists of 16 g of oxygen for every 1 g of hydrogen.
C) The seat you are sitting on is made up of atoms.
D) Hydrogen ignites in an open flame but helium does not.
E) The relative mass of a carbon atom is about 12 times that of hydrogen.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
26
Write the symbol for carbon. ______________

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
27
Co is the symbol for
A) carbon
B) cobalt
C) carbon monoxide
D) carboxide
E) chlorine
A) carbon
B) cobalt
C) carbon monoxide
D) carboxide
E) chlorine

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
28
Write the symbol for calcium. ______________

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
29
The total number of oxygen atoms indicated by the formula Cu3(PO4)2 is
A) 8
B) 4
C) 12
D) 16
E) 11
A) 8
B) 4
C) 12
D) 16
E) 11

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
30
The symbol for nitrogen is
A) Na
B) N
C) Ng
D) Ni
E) none of these
A) Na
B) N
C) Ng
D) Ni
E) none of these

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
31
How many of the following did Dalton not discuss in his atomic theory? isotopes
Ions
Protons
Electrons
Neutrons
A) 1
B) 2
C) 3
D) 4
E) 5
Ions
Protons
Electrons
Neutrons
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
32
In chemistry, a formula is used to represent
A) a compound
B) an element
C) a metal
D) all of these
E) none of these
A) a compound
B) an element
C) a metal
D) all of these
E) none of these

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
33
The symbol for the element mercury is
A) Hg
B) Mg
C) He
D) Mn
E) none of these
A) Hg
B) Mg
C) He
D) Mn
E) none of these

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
34
How many hydrogen atoms are indicated by the formula (NH4)2C6H8O2?
A) 16
B) 8
C) 12
D) 14
E) 24
A) 16
B) 8
C) 12
D) 14
E) 24

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
35
The symbol for copper is
A) Cr
B) C
C) Co
D) Cu
E) none of these
A) Cr
B) C
C) Co
D) Cu
E) none of these

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
36
Write the symbol for cobalt. ______________

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
37
How many atoms are represented by one formula unit of aluminum chromate, Al2(CrO4)3?
A) 17
B) 15
C) 21
D) 3
E) none of these
A) 17
B) 15
C) 21
D) 3
E) none of these

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
38
Write the symbol for lead. ______________

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
39
Si is the symbol for
A) silver
B) silicon
C) selenium
D) sodium
E) sulfur
A) silver
B) silicon
C) selenium
D) sodium
E) sulfur

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
40
Write the name for Ag. ______________

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
41
How many atoms of oxygen are in one formula unit of calcium dihydrogen phosphate?
A) 8
B) 4
C) 6
D) 9
E) 7
A) 8
B) 4
C) 6
D) 9
E) 7

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
42
Which particle has the smallest mass?
A) neutron
B) proton
C) electron
D) helium nucleus
A) neutron
B) proton
C) electron
D) helium nucleus

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
43
Which atomic particle determines the chemical behavior of an atom?
A) proton
B) electron
C) neutron
D) nucleus
E) none of these
A) proton
B) electron
C) neutron
D) nucleus
E) none of these

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
44
How many nitrogen atoms are indicated by the formula Al(NO2)3?
A) 3
B) 1
C) 6
D) 4
E) 0
A) 3
B) 1
C) 6
D) 4
E) 0

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
45
The first scientist to develop the theory that all atoms of a given element are identical was
A) John Dalton
B) J. J. Thomson
C) Lord Kelvin
D) Ernest Rutherford
E) James Chadwick
A) John Dalton
B) J. J. Thomson
C) Lord Kelvin
D) Ernest Rutherford
E) James Chadwick

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
46
An element's most stable ion forms an ionic compound with chlorine having the formula XCl2. If the ion of element X has a mass of 89 and 36 electrons, what is the identity of the element, and how many neutrons does it have?
A) Kr, 53 neutrons
B) Kr, 55 neutrons
C) Se, 55 neutrons
D) Sr, 51 neutrons
E) Rb, 52 neutrons
A) Kr, 53 neutrons
B) Kr, 55 neutrons
C) Se, 55 neutrons
D) Sr, 51 neutrons
E) Rb, 52 neutrons

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
47
How many protons, electrons, and neutrons, respectively, does 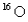 have?
have?
A) 8, 18, 8
B) 8, 8, 8
C) 8, 10, 8
D) 8, 14, 8
E) 8, 18, 16
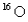 have?
have?A) 8, 18, 8
B) 8, 8, 8
C) 8, 10, 8
D) 8, 14, 8
E) 8, 18, 16

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following statements is/are true? I. John Dalton provided the first experimental support for the atom.
II) Ernest Rutherford proved the existence of a nucleus by shooting positively charged particles at a thin gold-foil sheet.
III) J. J. Thompson proved that electrons travel in elliptical orbits around the nucleus.
IV) Energy in an atom is quantized, so when electrons fall to their ground state, white light can be observed.
A) I, II
B) I, III
C) I, II, III
D) II, IV
E) All of the above statements (I - IV) are correct.
II) Ernest Rutherford proved the existence of a nucleus by shooting positively charged particles at a thin gold-foil sheet.
III) J. J. Thompson proved that electrons travel in elliptical orbits around the nucleus.
IV) Energy in an atom is quantized, so when electrons fall to their ground state, white light can be observed.
A) I, II
B) I, III
C) I, II, III
D) II, IV
E) All of the above statements (I - IV) are correct.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
49
The total number of atoms represented by the formula Co3(PO4)2 is
A) 13
B) 11
C) 8
D) 17
E) 6
A) 13
B) 11
C) 8
D) 17
E) 6

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following statements are true? I. The number of protons in an element is the same for all neutral atoms of that element.
II) The number of electrons in an element is the same for all neutral atoms of that element.
III) The number of neutrons in an element is the same for all neutral atoms of that element.
A) I, II, and III are all true.
B) Only I and II are true.
C) Only II and III are true.
D) Only I and III are true.
E) I, II, and III are all false.
II) The number of electrons in an element is the same for all neutral atoms of that element.
III) The number of neutrons in an element is the same for all neutral atoms of that element.
A) I, II, and III are all true.
B) Only I and II are true.
C) Only II and III are true.
D) Only I and III are true.
E) I, II, and III are all false.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
51
The chemical formula Al2O3 indicates
A) two atoms of aluminum and three atoms of oxygen
B) three atoms of aluminum and two atoms of oxygen
C) six atoms of each element
D) five atoms of each element
E) None of these is correct.
A) two atoms of aluminum and three atoms of oxygen
B) three atoms of aluminum and two atoms of oxygen
C) six atoms of each element
D) five atoms of each element
E) None of these is correct.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
52
How many phosphorus atoms are represented by one formula unit of cobalt(II) phosphate, Co3(PO4)2?
A) 2
B) 4
C) 6
D) 8
E) 1
A) 2
B) 4
C) 6
D) 8
E) 1

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
53
The chemical formula Ga2O3 indicates
A) two atoms of gallium and three atoms of oxygen
B) three atoms of gallium and two atoms of oxygen
C) six atoms of each element
D) five atoms of each element
E) none of these
A) two atoms of gallium and three atoms of oxygen
B) three atoms of gallium and two atoms of oxygen
C) six atoms of each element
D) five atoms of each element
E) none of these

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
54
The number of neutrons in one atom of 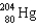 is
is
A) 124
B) 80
C) 204
D) 284
E) none of these
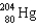 is
isA) 124
B) 80
C) 204
D) 284
E) none of these

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
55
How many protons, electrons, and neutrons, respectively, does 45Sc2+ have?
A) 21, 19, 24
B) 21, 21, 24
C) 19, 21, 26
D) 21, 19, 45
E) 19, 21, 24
A) 21, 19, 24
B) 21, 21, 24
C) 19, 21, 26
D) 21, 19, 45
E) 19, 21, 24

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
56
List the three main subatomic particles.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following contains the largest number of oxygen atoms?
A) 4H2O
B) 3CO2
C) O3
D) H2SO4
E) Al(NO3)3
A) 4H2O
B) 3CO2
C) O3
D) H2SO4
E) Al(NO3)3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
58
Mendeleev is credited with the modern atomic theory of atoms.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
59
An atom with 15 protons and 16 neutrons is an atom of
A) P
B) Ga
C) S
D) Pd
E) Rh
A) P
B) Ga
C) S
D) Pd
E) Rh

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
60
How many protons, electrons, and neutrons, respectively, does 31P have?
A) 15, 15, 16
B) 15, 16, 15
C) 16, 15, 31
D) 15, 15, 31
E) 15, 31, 16
A) 15, 15, 16
B) 15, 16, 15
C) 16, 15, 31
D) 15, 15, 31
E) 15, 31, 16

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
61
How many electrons are present in a bromine atom with a mass number of 87?
A) 35
B) 87
C) 122
D) 52
E) 6.02 *1023
A) 35
B) 87
C) 122
D) 52
E) 6.02 *1023

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
62
Which of these are isotopes of hydrogen?
A) "12C and 13C"
B) "2H and He"
C) "42K and 40K"
D) "3H and 2H"
E) "Li+ and He"
A) "12C and 13C"
B) "2H and He"
C) "42K and 40K"
D) "3H and 2H"
E) "Li+ and He"

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
63
A certain isotope X2+ contains 37 electrons and 73 neutrons. What is the mass number for this element?
A) 112
B) 110
C) 108
D) 39
E) 35
A) 112
B) 110
C) 108
D) 39
E) 35

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
64
How many neutrons are there in one atom of 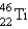 ?
?
A) 22
B) 24
C) 46
D) 68
E) none of these
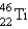 ?
?A) 22
B) 24
C) 46
D) 68
E) none of these

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
65
The average mass of a boron atom is 10.81. Assuming you were able to isolate only one boron atom, the chance that you would randomly get one with a mass of 10.81 is
A) 0%
B) 0.81%
C) about 11%
D) 10.81%
E) greater than 50%
A) 0%
B) 0.81%
C) about 11%
D) 10.81%
E) greater than 50%

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
66
The atom with 69 neutrons and 50 protons has a mass number of
A) 69
B) 50
C) 19
D) 119
E) cannot be determined from information given
A) 69
B) 50
C) 19
D) 119
E) cannot be determined from information given

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
67
An atom that has 54 protons, 54 electrons, and 78 neutrons is
A)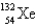
B)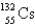
C)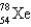
D)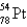
E)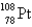
A)
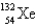
B)
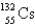
C)
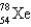
D)
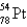
E)
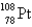

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
68
The mass number of an atom equals
A) the number of neutrons per atom
B) the atomic mass of the element
C) the atomic number of the element
D) the number of protons plus the number of neutrons per atom
E) none of the above
A) the number of neutrons per atom
B) the atomic mass of the element
C) the atomic number of the element
D) the number of protons plus the number of neutrons per atom
E) none of the above

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
69
Determine the number of protons and neutrons in Cu-63.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
70
The number of protons in the nucleus of an atom is called its
A) mass number
B) valence
C) isotope number
D) atomic number
E) none of these
A) mass number
B) valence
C) isotope number
D) atomic number
E) none of these

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
71
Determine the number of protons and the number of neutrons in an atom of Sn-118.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
72
Identify each of the following.
a. The heaviest noble gas
b. The transition metal that has 24 electrons as a +3 ion
c. The halogen in the third period
d. The alkaline earth metal that has 18 electrons as a stable ion
a. The heaviest noble gas
b. The transition metal that has 24 electrons as a +3 ion
c. The halogen in the third period
d. The alkaline earth metal that has 18 electrons as a stable ion

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
73
Give the symbol and number of neutrons for an element with atomic number 26 and mass number 56.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
74
Choose the pair in which the components have the same charge.
A) a proton and a hydrogen atom
B) a hydrogen atom and an electron
C) a neutron and a hydrogen atom
D) an electron and a neutron
A) a proton and a hydrogen atom
B) a hydrogen atom and an electron
C) a neutron and a hydrogen atom
D) an electron and a neutron

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
75
Which pair have approximately the same mass?
A) a hydrogen,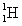 , and a deuterium,
, and a deuterium, 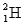 , atom
, atom
B) a neutron and an electron
C) a proton and a neutron
D) an electron and a proton
A) a hydrogen,
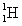 , and a deuterium,
, and a deuterium, 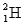 , atom
, atomB) a neutron and an electron
C) a proton and a neutron
D) an electron and a proton

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
76
Determine the number of protons and the number of neutrons in an atom of Rb-85.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
77
The number of protons in 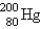 is
is
A) 80
B) 120
C) 200
D) dependent on ionic charge
E) unknown
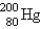 is
isA) 80
B) 120
C) 200
D) dependent on ionic charge
E) unknown

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
78
How many neutrons are contained in an iodine nucleus with a mass number of 131?
A) 53
B) 74
C) 78
D) 127
E) 131
A) 53
B) 74
C) 78
D) 127
E) 131

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
79
How many electrons are present in a tin atom with a mass number of 119?
A) 50
B) 119
C) 169
D) 69
E) 6.02 *1023
A) 50
B) 119
C) 169
D) 69
E) 6.02 *1023

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
80
The number of neutrons in 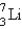 is
is
A) 10
B) 9
C) 7
D) 4
E) 3
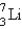 is
isA) 10
B) 9
C) 7
D) 4
E) 3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck


