Deck 17: Reactions of Aromatic Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/129
Play
Full screen (f)
Deck 17: Reactions of Aromatic Compounds
1
Which of the following is the best choice of reagents to effect the electrophilic iodination of an aromatic ring?
A) KI, acetone
B) I2, CH3CN
C) KI, HNO3
D) I2, HNO3
E) none of the above
A) KI, acetone
B) I2, CH3CN
C) KI, HNO3
D) I2, HNO3
E) none of the above
I2, HNO3
2
Provide the necessary reagents to accomplish the following transformation. 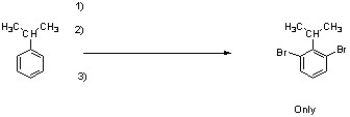

1) SO3 / H2SO4
2) excess Br2 / FeBr3
3) H+, H2O, heat
2) excess Br2 / FeBr3
3) H+, H2O, heat
3
Which of the following is the strongest activating group in electrophilic aromatic substitution reactions?
A) -CH2CH3
B) -OCH3
C) -CO2CH3
D) -NO2
E) -N(CH3)2
A) -CH2CH3
B) -OCH3
C) -CO2CH3
D) -NO2
E) -N(CH3)2
-N(CH3)2
4
In electrophilic aromatic substitution reactions the hydroxyl group is an o,p-director because:
A) it donates electron density to the ring by induction and destabilizes the meta sigma complex.
B) it donates electron density to the ring by resonance and stabilizes the ortho and para sigma complexes.
C) it donates electron density to the ring by induction and stabilizes the ortho and para sigma complexes.
D) it donates electron density to the ring by resonance and destabilizes the meta sigma complex.
E) it withdraws electron density from the ring by induction and destabilizes the meta sigma complex.
A) it donates electron density to the ring by induction and destabilizes the meta sigma complex.
B) it donates electron density to the ring by resonance and stabilizes the ortho and para sigma complexes.
C) it donates electron density to the ring by induction and stabilizes the ortho and para sigma complexes.
D) it donates electron density to the ring by resonance and destabilizes the meta sigma complex.
E) it withdraws electron density from the ring by induction and destabilizes the meta sigma complex.

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following species is attacked by benzene in the electrophilic nitration reaction?
A) HNO3
B) NO2+
C) NO2
D) NO+
E) N3-
A) HNO3
B) NO2+
C) NO2
D) NO+
E) N3-

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
6
Under what reaction conditions does the electrophilic chlorination of aromatic compounds usually occur?
A) Cl2, AlCl3
B) Cl2, H2O
C) Cl2, CCl4
D) NaCl, H2O
E) NaCl, CH3OH
A) Cl2, AlCl3
B) Cl2, H2O
C) Cl2, CCl4
D) NaCl, H2O
E) NaCl, CH3OH

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
7
Provide the structure of the major organic product(s) in the following reaction. 


Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
8
In electrophilic aromatic substitution reactions, a -NHCOCH3 substituent on the aromatic ring is:
A) a deactivator and a m-director.
B) a deactivator and an o,p-director.
C) an activator and a m-director.
D) an activator and an o,p-director.
E) none of the above.
A) a deactivator and a m-director.
B) a deactivator and an o,p-director.
C) an activator and a m-director.
D) an activator and an o,p-director.
E) none of the above.

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
9
In the bromination of benzene using Br2 and FeBr3, is the intermediate carbocation aromatic? Explain.

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
10
Which step in an electrophilic aromatic substitution reaction is typically rate determining, formation of the sigma complex or loss of H+ by the sigma complex to form the product? Explain.

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following compounds will undergo bromination most rapidly using Br2, FeBr3?
A) p-methylacetanilide
B) bromobenzene
C) acetanilide
D) benzenesulfonic acid
E) dibromobenzene
A) p-methylacetanilide
B) bromobenzene
C) acetanilide
D) benzenesulfonic acid
E) dibromobenzene

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is an incorrect statement about the bromination of benzene by Br2 and FeBr3?
A) FeBr3 functions to increase the electrophilicity of Br2.
B) Formation of the sigma complex is the rate-determining step of the mechanism.
C) The carbanionic intermediate is resonance stabilized.
D) There are two carbon-containing intermediates in the mechanism.
E) The final step of the mechanism is loss of H+.
A) FeBr3 functions to increase the electrophilicity of Br2.
B) Formation of the sigma complex is the rate-determining step of the mechanism.
C) The carbanionic intermediate is resonance stabilized.
D) There are two carbon-containing intermediates in the mechanism.
E) The final step of the mechanism is loss of H+.

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
13
In electrophilic aromatic substitution reactions a bromine substituent:
A) is a deactivator and a m-director.
B) is a deactivator and an o,p-director.
C) is an activator and a m-director.
D) is an activator and an o,p-director.
E) none of the above
A) is a deactivator and a m-director.
B) is a deactivator and an o,p-director.
C) is an activator and a m-director.
D) is an activator and an o,p-director.
E) none of the above

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
14
Provide the major resonance structures of the intermediate sigma complex in the reaction of benzene with the generic electrophile E+.

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
15
Which sequence correctly ranks the following aromatic rings in order of increasing rate of reactivity with chlorine and aluminum chloride? 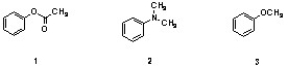
A) 1 < 2 < 3
B) 2 < 3 < 1
C) 3 < 2 < 1
D) 2 < 1 < 3
E) 1 < 3 < 2

A) 1 < 2 < 3
B) 2 < 3 < 1
C) 3 < 2 < 1
D) 2 < 1 < 3
E) 1 < 3 < 2

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
16
Provide a detailed, stepwise mechanism for the reaction of benzene with Br2 and FeBr3. Make sure to include the activating reaction between Br2 and FeBr3 in your mechanism.

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following compounds will react most rapidly when treated with CH3CH2Cl and AlCl3?
A) benzene
B) chlorobenzene
C) nitrobenzene
D) anisole
E) toluene
A) benzene
B) chlorobenzene
C) nitrobenzene
D) anisole
E) toluene

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following compounds will undergo Friedel-Crafts alkylation with (CH3)3CCl, AlCl3 most rapidly?
A) toluene
B) iodobenzene
C) acetophenone
D) benzenesulfonic acid
E) cyanobenzene
A) toluene
B) iodobenzene
C) acetophenone
D) benzenesulfonic acid
E) cyanobenzene

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
19
Electrophilic iodination of benzene requires which reagent in addition to I2?

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
20
Name the major organic product which results when 3-ethylbenzenesulfonic acid is heated in aqueous acid.

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
21
Provide the structure of the major organic product(s) in the following reaction. 

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
22
In electrophilic aromatic substitution reactions, a phenyl substituent on the aromatic ring is:
A) a deactivator and a m-director.
B) a deactivator and an o,p-director.
C) an activator and a m-director.
D) an activator and an o,p-director.
E) none of the above.
A) a deactivator and a m-director.
B) a deactivator and an o,p-director.
C) an activator and a m-director.
D) an activator and an o,p-director.
E) none of the above.

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
23
Which sequence correctly ranks the following aromatic rings in order of increasing rate of reactivity in an electrophilic aromatic substitution reaction? 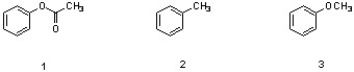
A) 1 < 2 < 3
B) 2 < 3 < 1
C) 3 < 1 < 2
D) 2 < 1 < 3

A) 1 < 2 < 3
B) 2 < 3 < 1
C) 3 < 1 < 2
D) 2 < 1 < 3

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
24
Rank the following compounds in order of increasing reactivity towards chlorination with Cl2/AlCl3 (slowest reacting to fastest). 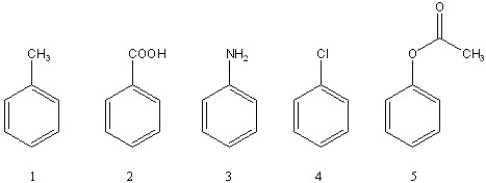
A) 3 < 4 < 2 < 1 < 5
B) 2 < 4 < 1 < 3 < 5
C) 4 < 2 < 1 < 3 < 5
D) 2 < 4 < 5 < 1 < 3
E) 2 < 4 < 1 < 5 < 3

A) 3 < 4 < 2 < 1 < 5
B) 2 < 4 < 1 < 3 < 5
C) 4 < 2 < 1 < 3 < 5
D) 2 < 4 < 5 < 1 < 3
E) 2 < 4 < 1 < 5 < 3

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
25
In the addition of an electrophile to acetophenone, which of the following best describes the expected mode of reaction?
A) The o,p-positions are most activated to attack by the electrophile.
B) The m-positions are most activated to attack by the electrophile.
C) The o,p-positions are most deactivated to attack by the electrophile.
D) The m-positions are most deactivated to attack by the electrophile.
E) All positions (o, m, and p) are equally activated to attack by the electrophile.
A) The o,p-positions are most activated to attack by the electrophile.
B) The m-positions are most activated to attack by the electrophile.
C) The o,p-positions are most deactivated to attack by the electrophile.
D) The m-positions are most deactivated to attack by the electrophile.
E) All positions (o, m, and p) are equally activated to attack by the electrophile.

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
26
Provide the structure of the major mononitration product of the compound below. 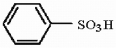


Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
27
Provide the major organic product of the following reaction. 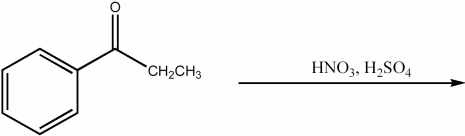


Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
28
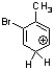
Which of the following is an intermediate in the bromination of toluene?
A)
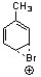
B)
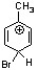
C)
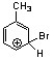
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
29
Based upon the following energy profile diagram for the first step of an electrophilic aromatic substitution reaction, which of the following substituents is X mostly likely to be? 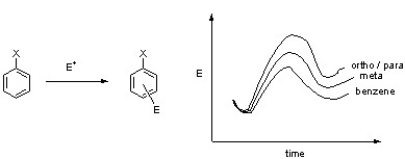
A) Cl
B) OCH3
C) CO2H
D) CH3

A) Cl
B) OCH3
C) CO2H
D) CH3

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
30
Derivatives of the compound shown below are currently being examined for their effectiveness in treating drug addiction and metabolic syndrome (J. Med. Chem. 2006, 872). Which sequence ranks the following aromatic rings of this compound in order of increasing reactivity in an electrophilic aromatic substitution reaction (slowest to fastest reacting)? 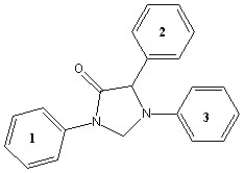
A) 1 < 2 < 3
B) 2 < 3 < 1
C) 3 < 2 < 1
D) 3 < 1 < 2
E) 2 < 1 < 3

A) 1 < 2 < 3
B) 2 < 3 < 1
C) 3 < 2 < 1
D) 3 < 1 < 2
E) 2 < 1 < 3

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
31
In electrophilic aromatic substitution reactions, a cyano substituent on the aromatic ring is:
A) a deactivator and a m-director.
B) a deactivator and an o,p-director.
C) an activator and a m-director.
D) an activator and an o,p-director.
E) none of the above.
A) a deactivator and a m-director.
B) a deactivator and an o,p-director.
C) an activator and a m-director.
D) an activator and an o,p-director.
E) none of the above.

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
32
The nitration of anisole:
A) proceeds more rapidly than the nitration of benzene and yields predominantly the meta product.
B) proceeds more rapidly than the nitration of benzene and yields predominantly the ortho, para products.
C) proceeds more slowly than the nitration of benzene and yields predominantly the meta product.
D) proceeds more slowly than the nitration of benzene and yields predominantly the ortho, para products.
E) proceeds at the same rate as the nitration of benzene and yields predominantly the meta product.
A) proceeds more rapidly than the nitration of benzene and yields predominantly the meta product.
B) proceeds more rapidly than the nitration of benzene and yields predominantly the ortho, para products.
C) proceeds more slowly than the nitration of benzene and yields predominantly the meta product.
D) proceeds more slowly than the nitration of benzene and yields predominantly the ortho, para products.
E) proceeds at the same rate as the nitration of benzene and yields predominantly the meta product.

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
33
Provide the major organic product of the following reaction. 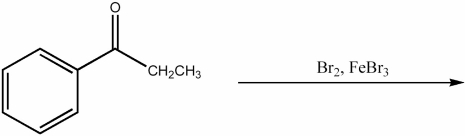


Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
34
In electrophilic aromatic substitution reactions the nitro group is:
A) a m-director since it destabilizes the meta sigma complex more than the ortho, para.
B) a m-director since it destabilizes the meta sigma complex less than the ortho, para.
C) an o,p-director since it stabilizes the ortho, para sigma complex more than the meta.
D) an o,p-director since it stabilizes the ortho, para sigma complex less than the meta.
E) none of the above.
A) a m-director since it destabilizes the meta sigma complex more than the ortho, para.
B) a m-director since it destabilizes the meta sigma complex less than the ortho, para.
C) an o,p-director since it stabilizes the ortho, para sigma complex more than the meta.
D) an o,p-director since it stabilizes the ortho, para sigma complex less than the meta.
E) none of the above.

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following compounds will react least rapidly when treated with CH3CH2Cl and AlCl3?
A) o-xylene
B) acetanilide
C) toluene
D) benzene
E) bromobenzene
A) o-xylene
B) acetanilide
C) toluene
D) benzene
E) bromobenzene

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following compounds will undergo bromination least rapidly when treated with Br2 and FeBr3?
A) p-methylacetanilide
B) bromobenzene
C) acetanilide
D) benzenesulfonic acid
E) benzene
A) p-methylacetanilide
B) bromobenzene
C) acetanilide
D) benzenesulfonic acid
E) benzene

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
37
Draw the three major resonance structures of the sigma complex intermediate in the reaction of acetophenone with HNO3/H2SO4 to yield o-nitroacetophenone. Circle the resonance form which is less stable than the other two.

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
38
Draw the four major resonance structures of the sigma complex intermediate in the reaction of anisole with HNO3/H2SO4 to yield p-nitroanisole.

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
39
In electrophilic aromatic substitution reactions, a -CO2H substituent on the aromatic ring is:
A) a deactivator and a m-director.
B) a deactivator and an o,p-director.
C) an activator and a m-director.
D) an activator and an o,p-director.
E) none of the above.
A) a deactivator and a m-director.
B) a deactivator and an o,p-director.
C) an activator and a m-director.
D) an activator and an o,p-director.
E) none of the above.

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following compounds will not undergo Friedel-Crafts acylation when treated with 
A) toluene
B) p-xylene
C) anisole
D) ethoxybenzene
E) benzophenone

A) toluene
B) p-xylene
C) anisole
D) ethoxybenzene
E) benzophenone

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
41
Provide the major organic product of the following reaction. 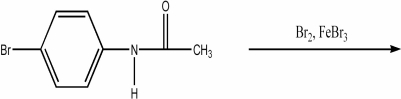


Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
42
Provide the major organic product of the following reaction. 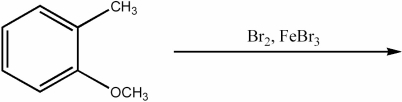
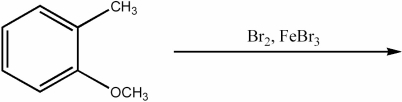

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
43
Provide the major organic product(s) when benzene is treated with the following sequence of reagents: 1. Br2, FeBr3 2. HNO3, H2SO4.

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
44
Provide the major organic product(s) of the reaction shown below. 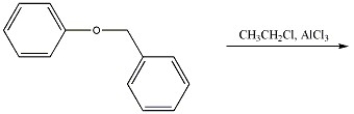


Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following compounds undergoes reaction with HNO3/H2SO4 at the fastest rate?
A) ethylbenzene
B) benzenesulfonic acid
C) nitrobenzene
D) chlorobenzene
E) acetophenone
A) ethylbenzene
B) benzenesulfonic acid
C) nitrobenzene
D) chlorobenzene
E) acetophenone

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
46
Provide the structure of the major organic product(s) in the following reaction. 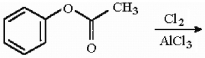


Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
47
Rank the following sigma complexes in order of increasing stability. 


Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
48
Provide the major organic product of the following reaction. 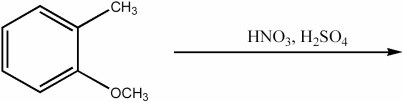
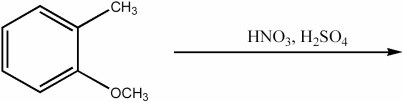

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
49
Rank the following groups in order of increasing activating power in electrophilic aromatic substitution reactions: -OCH3, -OCOCH2CH3, -CH2CH3, -Br.

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
50
Provide the major organic product(s) of the reaction shown below. 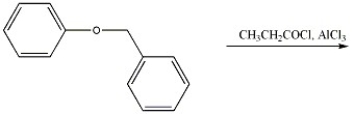


Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
51
Provide the major organic product of the following reaction. 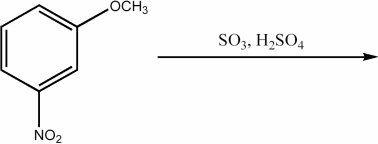


Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
52
Provide the major organic product(s) of the reaction shown below. 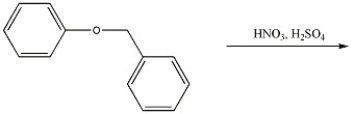


Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
53
Provide the major organic product(s) when benzene is treated with the following sequence of reagents: 1. Br2, FeBr3 2. CH3COCl, AlCl3.

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
54
Provide the major organic product of the following reaction. 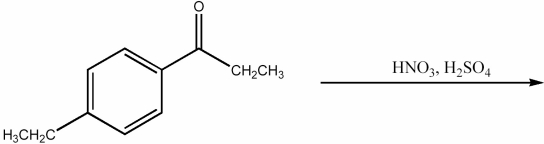


Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
55
Provide the major organic product(s) of the reaction shown below. 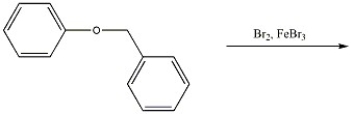


Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
56
Provide the major organic product of the following reaction. 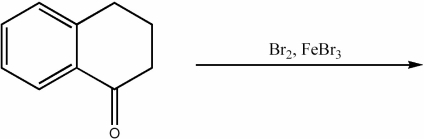


Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
57
Provide the structure of the most stable resonance form of the intermediate that yields p-bromoanisole when anisole is treated with Br2/FeBr3.

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
58
Provide the major organic product(s) of the reaction shown below. 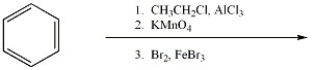
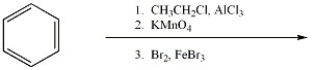

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
59
Provide the major organic product that results when benzene is treated with the following sequence of reagents: 1. HNO3, H2SO4 2. Br2, FeBr3.

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
60
Provide the structure of the major mononitration product(s) of the compound below. 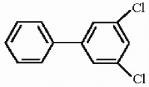


Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
61
Provide a series of synthetic steps by which the compound below can be prepared from benzene. 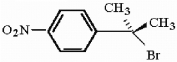


Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
62
Provide the major organic product of the following reaction. 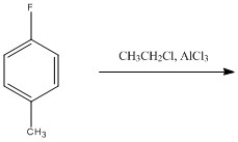


Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
63
Provide the major organic product of the following reaction. 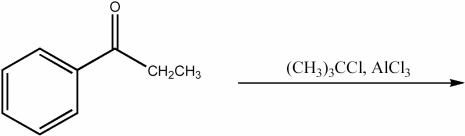


Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
64
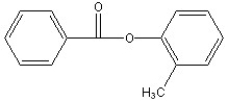
Predict the two major products from the following reaction. (Mark all correct responses.) 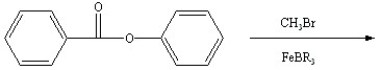
A)
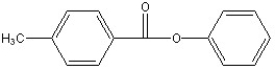
B)
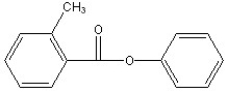
C)
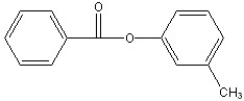
D)
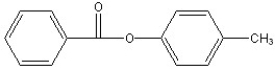
E)

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
65
Provide the structure of the major monoitration product of the compound below. 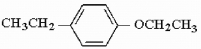


Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
66
List of the three limitations usually associated with the Friedel-Crafts alkylation reaction.

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
67
Provide the major organic product(s) of the reaction shown below. 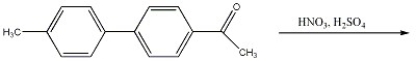


Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
68
Provide the structure of the major mononitration product of the compound below. 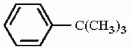


Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
69
Provide the major organic product of the following reaction. 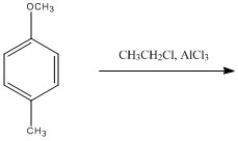


Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
70
What sequence of reagents is needed to convert benzene into m-bromoethylbenzene?

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
71
Provide the structure of the major organic product of the following reaction. 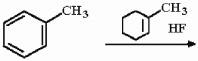


Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following reactions will actually yield the indicated product?
A)
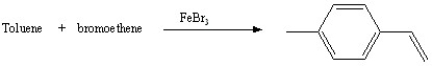
B)
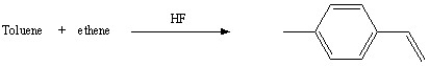
C)

D)
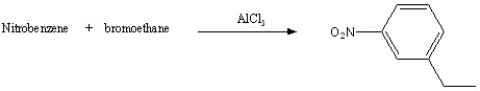
E)
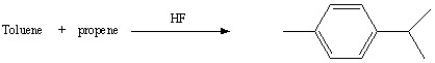
A)
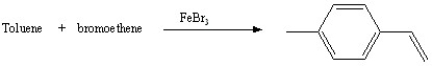
B)

C)

D)

E)


Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
73
Provide the major organic product of the following reaction. 


Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
74
Provide the structure of the major mononitration product of the compound below. 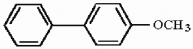


Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
75
Provide a detailed, stepwise mechanism for the following reactions. 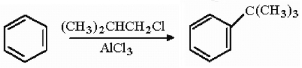


Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
76
Provide the major organic product of the following reaction. 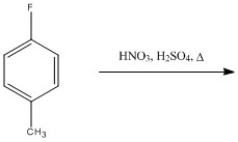


Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
77
Provide the major organic product of the following reaction. 


Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
78
Provide the structure of the major mononitration product(s) of the compound below. 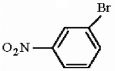


Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
79
Provide the major organic product that results when benzene is treated with the following sequence of reagents: 1. CH3COCl, AlCl3 2. Br2, FeBr3.

Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck
80
Provide the major organic product of the following reaction. 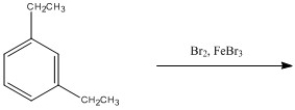


Unlock Deck
Unlock for access to all 129 flashcards in this deck.
Unlock Deck
k this deck


