Deck 26: Synthetic Polymers
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/128
Play
Full screen (f)
Deck 26: Synthetic Polymers
1
Which of the following is the best initiator for a free-radical polymerization?
A) BuLi
B) PhOH
C) (PhCO2)2
D) CH3CH(OCH3)2
E) BF3
A) BuLi
B) PhOH
C) (PhCO2)2
D) CH3CH(OCH3)2
E) BF3
(PhCO2)2
2
Which of the following is the best initiator for an anionic polymerization?
A) BuLi
B) PhOH
C) (PhCO2)2
D) CH3CH(OCH3)2
E) BF3
A) BuLi
B) PhOH
C) (PhCO2)2
D) CH3CH(OCH3)2
E) BF3
BuLi
3
________ polymers result from rapid reaction of one molecule at a time with a growing polymer chain, usually with a reactive intermediate at the growing end of the chain.
A) Addition
B) Condensation
C) Step-growth
D) Amorphous
A) Addition
B) Condensation
C) Step-growth
D) Amorphous
Addition
4
"Super" glues are typically composed of what polymer?
A) poly(acrylonitrile)
B) poly(methyl α-cyanoacrylate)
C) polystyrene
D) polypropylene
E) poly(methyl α-methylacrylate)
A) poly(acrylonitrile)
B) poly(methyl α-cyanoacrylate)
C) polystyrene
D) polypropylene
E) poly(methyl α-methylacrylate)

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
5
________ polymers result from polymerization reactions in which chain growth is accompanied by loss of a small molecule.
A) Addition
B) Condensation
C) Chain-growth
D) Latex
A) Addition
B) Condensation
C) Chain-growth
D) Latex

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
6
What monomer is needed to produce the polymer below? 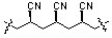


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
7
Provide the structure of the monomer from which polystyrene is formed.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
8
The monomer shown below, 1,1-bistrifluoromethylethylene, undergoes polymerization via: 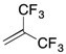
A) anionic polymerization
B) cationic polymerization
C) radical polymerization
D) chain-growth polymerization

A) anionic polymerization
B) cationic polymerization
C) radical polymerization
D) chain-growth polymerization

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
9
Plexiglas is a:
A) polyamide.
B) polyester.
C) polycarbonate.
D) polyurethane.
E) none of the above
A) polyamide.
B) polyester.
C) polycarbonate.
D) polyurethane.
E) none of the above

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
10
Is the polymer shown an addition polymer or a condensation polymer? 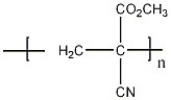


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
11
Provide the structure of the monomer from which Teflon is formed.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is not an addition polymer?
A) polyethylene
B) Orlon
C) Plexiglas
D) Kevlar
E) Teflon
A) polyethylene
B) Orlon
C) Plexiglas
D) Kevlar
E) Teflon

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
13
Provide the structure of poly(isobutylene).

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following monomers is most well-suited to cationic polymerization?
A) isobutylene
B) ethylene
C) acrylonitrile
D) methyl α-methacrylate
E) methyl α-cyanoacrylate
A) isobutylene
B) ethylene
C) acrylonitrile
D) methyl α-methacrylate
E) methyl α-cyanoacrylate

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
15
What is the carbon : hydrogen mole ratio in polyethylene?
A) 12:1
B) 1:2
C) 1:3
D) 3:2
E) 1:100
A) 12:1
B) 1:2
C) 1:3
D) 3:2
E) 1:100

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
16
Ethylene oxide can be polymerized to form Carbowax. What conditions are best for the polymerization of ethylene oxide?
A) anionic
B) cationic
C) free-radical
D) carbene
E) none of the above
A) anionic
B) cationic
C) free-radical
D) carbene
E) none of the above

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following is the best initiator for a cationic polymerization?
A) BuLi
B) PhOH
C) (PhCO2)2
D) CH3CH(OCH3)2
E) BF3
A) BuLi
B) PhOH
C) (PhCO2)2
D) CH3CH(OCH3)2
E) BF3

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
18
Draw the structure of poly(acrylonitrile).

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
19
From which of the following are water pipes commonly manufactured?
A) poly(methyl α-cyanoacrylate)
B) polyurethane
C) poly(acrylonitrile)
D) poly(vinyl chloride)
E) poly(isobutylene)
A) poly(methyl α-cyanoacrylate)
B) polyurethane
C) poly(acrylonitrile)
D) poly(vinyl chloride)
E) poly(isobutylene)

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
20
Provide the structure of the monomer from which polypropylene is formed.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
21
When ethylene is polymerized by free-radical initiation, what type of polyethylene is formed?
A) low-density; highly branched
B) low-density; unbranched and highly linear
C) high-density; highly branched
D) high-density; unbranched and highly linear
E) high-density; highly crystalline
A) low-density; highly branched
B) low-density; unbranched and highly linear
C) high-density; highly branched
D) high-density; unbranched and highly linear
E) high-density; highly crystalline

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
22
Propose a synthesis of poly(vinyl alcohol) from 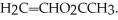
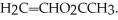

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
23
Provide a mechanism to show how H2C 
C(CH3)2 is polymerized using BF3 as the initiator.

C(CH3)2 is polymerized using BF3 as the initiator.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
24
Super glue shown below is most likely constructed under what type of mechanism? 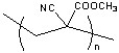
A) radical
B) cationic
C) anionic
D) condensation

A) radical
B) cationic
C) anionic
D) condensation

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
25
Provide the structure of Teflon.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
26
The mechanism by which poly(methyl α-cyanoacrylate) formed was most likely ________.
A) anionic addition
B) cationic addition
C) radical addition
D) condensation
A) anionic addition
B) cationic addition
C) radical addition
D) condensation

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
27
Draw the structure of the polymer produced in the following reaction.
CH3CH
CH2
CH3CH

CH2

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
28
Provide the structure of the monomer from which 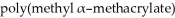
is formed.
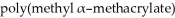
is formed.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
29
Explain why H2C 
CHCO2CH3 does not undergo cationic polymerization.

CHCO2CH3 does not undergo cationic polymerization.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
30
In polymerization reactions what purpose does benzoyl peroxide usually serve?

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
31
Show how branching could occur during the free-radical polymerization of styrene.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
32
Provide the structure of the monomer from which poly(vinyl chloride) is formed.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following is the monomer unit of the polymer shown below? 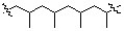
A)
B)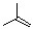
C)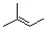
D)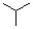

A)
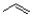
B)

C)

D)


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following monomers is least readily polymerized under conditions of anionic polymerization?
A) isobutylene
B) acrylonitrile
C) methyl acrylate
D) methyl α-cyanoacrylate
E) methyl α-methacrylate
A) isobutylene
B) acrylonitrile
C) methyl acrylate
D) methyl α-cyanoacrylate
E) methyl α-methacrylate

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
35
Styrene undergoes polymerization via what type of mechanism?
A) anionic polymerization
B) cationic polymerization
C) radical polymerization
D) It will polymerize via all three mechanisms.
A) anionic polymerization
B) cationic polymerization
C) radical polymerization
D) It will polymerize via all three mechanisms.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
36
Provide the structure of the monomer from which poly(acrylonitrile) is formed.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
37
Predict the monomer units that combine to form polyurethane. 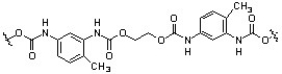


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following is least readily formed by cationic polymerization?
A) poly(acrylonitrile)
B) poly(isobutylene)
C) poly(propylene)
D) polystyrene
E) All of the above have equal propensity to undergo cationic polymerization.
A) poly(acrylonitrile)
B) poly(isobutylene)
C) poly(propylene)
D) polystyrene
E) All of the above have equal propensity to undergo cationic polymerization.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
39
Poly (vinyl acetate) is used in the backing of carpet, and in paints and adhesives. Provide two repeating units of poly (vinyl acetate).

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
40
Show each step in the mechanism for the following transformation. Include all intermediates and be sure to use arrows correctly to indicate placement of electrons. 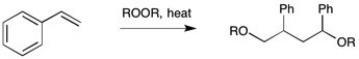


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
41
An alternating copolymer is shown below. Is this an addition polymer or a condensation polymer? 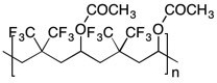


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
42
If the side groups of a polymer occur randomly on either side of the polymer backbone, then the polymer is called ________.
A) atactic
B) syndiotactic
C) isotactic
D) enantiotactic
E) randiotactic
A) atactic
B) syndiotactic
C) isotactic
D) enantiotactic
E) randiotactic

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
43
Draw three repeating units of poly(vinyl chloride), having syndiotactic stereochemistry.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
44
Maleic anhydride (below) undergoes chain-growth polymerization but not step-growth polymerizations. Why not? 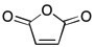


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
45
Maleic anhydride (below) undergoes addition polymerization. Draw the structure of the polymer that results. 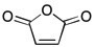


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
46
An alternating copolymer is shown below. What two monomers were used to make this polymer? 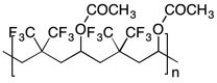


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
47
Why can't poly(vinyl alcohol) be synthesized directly from vinyl alcohol as shown below? 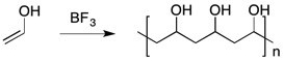


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
48
Provide the structure of isotactic polystyrene.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
49
Show the reaction which occurs when benzoyl peroxide is heated.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
50
Provide the structure of syndiotactic polystyrene.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
51
If the side groups of a polymer chain are generally on the same side of the polymer backbone, the polymer is called ________.
A) atactic
B) syndiotactic
C) transiotactic
D) cisisotactic
E) isotactic
A) atactic
B) syndiotactic
C) transiotactic
D) cisisotactic
E) isotactic

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
52
Provide the structure of isotactic polypropylene.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
53
Provide a representation of atactic poly(acrylonitrile).

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
54
Why is chain branching less common in anionic polymerization than in cationic polymerization?

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
55
Provide a mechanism to show how H2C 
CHCO2CH3 is polymerized using butyllithium as the initiator.

CHCO2CH3 is polymerized using butyllithium as the initiator.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
56
Provide the structure of atactic polypropylene.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
57
Provide the structure of syndiotactic polypropylene.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
58
Neoprene is a flexible, chemically resistant polymer used in shoe soles, hoses and wetsuits. Based on the polymer structure of neoprene, provide a structure for the monomer of neoprene. 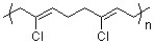


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
59
Provide the structure of atactic polystyrene.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
60
Provide a representation of isotactic poly(acrylonitrile).

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following polymers can be classified as copolymers? 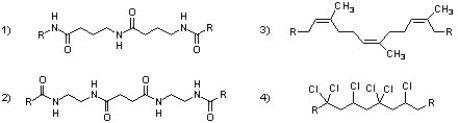
A) 1 & 2
B) 2 & 4
C) 1 & 4
D) 2 & 3
E) 1, 3, & 4

A) 1 & 2
B) 2 & 4
C) 1 & 4
D) 2 & 3
E) 1, 3, & 4

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
62
Provide the structure of natural rubber.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
63
Polymers composed of identical monomer units are known as ________.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
64
Another name for a urethane is a(n) ________.
A) carbamate ester
B) carbonate ester
C) orthoester
D) aryl diisocyanate
E) carbamic acid
A) carbamate ester
B) carbonate ester
C) orthoester
D) aryl diisocyanate
E) carbamic acid

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
65
What type of polymer is formed from ethylene glycol and a diester as monomer components?
A) polyurethanes
B) polyamides
C) polyesters
D) polycarbonates
E) none of the above
A) polyurethanes
B) polyamides
C) polyesters
D) polycarbonates
E) none of the above

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
66
How does vulcanization of rubber alter its structure and properties?

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
67
Provide the structure of Saran, an alternating copolymer of 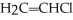
and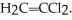
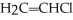
and
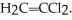

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
68
Named for a pair of Nobel Prize winners, ________ catalysts, which are composed of aluminum- and titanium-containing Lewis acids, provide a high measure of stereocontrol in alkene polymerizations.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
69
What substance results from the 1,4-polymerization of 1,3-butadiene?

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
70
Draw the structure of the polymer produced in the following reaction. 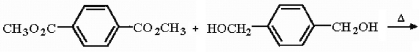
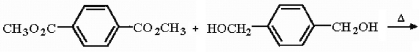

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
71
Cis-1,4-Polybutadiene is one form of synthetic rubber. Provide the structure of this polymer.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
72
Dacron is a ________.
A) polyester
B) polyamide
C) polycarbonate
D) polyurethane
E) none of the above
A) polyester
B) polyamide
C) polycarbonate
D) polyurethane
E) none of the above

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
73
The stereochemistry observed in this polymer is described as ________. 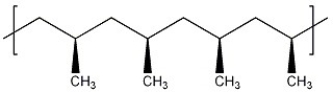
A) atactic
B) isotactic
C) syndiotactic
D) tacky
E) regiotactic

A) atactic
B) isotactic
C) syndiotactic
D) tacky
E) regiotactic

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
74
What are copolymers?

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
75
Draw the structure of the polymer produced in the following reaction. 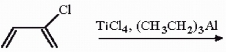
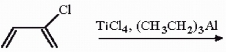

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
76
Natural rubber is a ________.
A) polyamide
B) polyester
C) polycarbonate
D) polyurethane
E) none of the above
A) polyamide
B) polyester
C) polycarbonate
D) polyurethane
E) none of the above

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
77
Shown below is a segment of polypropylene. Which of the following terms describes the stereochemistry of the methyl substituents? 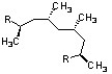
A) atactic
B) isotactic
C) syndiotactic
D) tacky
E) peritactic

A) atactic
B) isotactic
C) syndiotactic
D) tacky
E) peritactic

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
78
The structure of cellulose is shown below. What type of polymer is it? 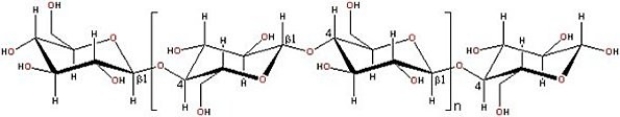
A) Alternating copolymer
B) Chain-growth polymer
C) Step-growth polymer
D) Ziegler-Natta polymer

A) Alternating copolymer
B) Chain-growth polymer
C) Step-growth polymer
D) Ziegler-Natta polymer

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following elements is necessary for the vulcanization of rubber?
A) sulfur
B) silicon
C) phosphorus
D) boron
E) titanium
A) sulfur
B) silicon
C) phosphorus
D) boron
E) titanium

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
80
Nylon 6 is a ________.
A) polyurethane
B) polyester
C) polycarbonate
D) polyamide
E) none of the above
A) polyurethane
B) polyester
C) polycarbonate
D) polyamide
E) none of the above

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck


