Deck 20: Carboxylic Acid Derivatives
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/44
Play
Full screen (f)
Deck 20: Carboxylic Acid Derivatives
1
What would be the major organic product of the following reaction? 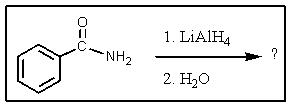
A)
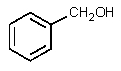
B)
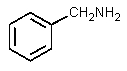
C)
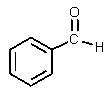
D)
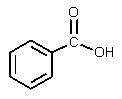
E)
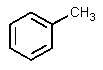

A)

B)

C)

D)

E)


2
What would account for the fact that the molecule below racemizes easily,despite having three chiral centers? 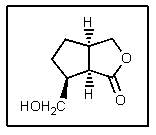
A) Lactones are configurationally unstable
B) Molecule loses CO2 easily
C) Enolization occurs easily
D) Intramolecular transesterification occurs easily
E) This molecule is not chiral.

A) Lactones are configurationally unstable
B) Molecule loses CO2 easily
C) Enolization occurs easily
D) Intramolecular transesterification occurs easily
E) This molecule is not chiral.
Intramolecular transesterification occurs easily
3
What would be the organic product of the following reaction? 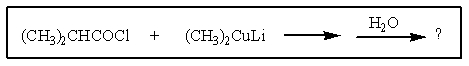
A)
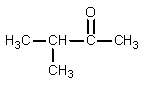
B)
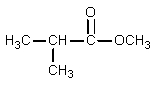
C)
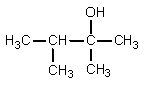
D)
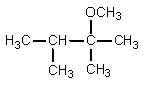
E) None of these.
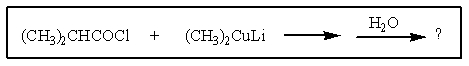
A)

B)

C)

D)

E) None of these.

4
Rank the following carboxylic acid derivatives in order of decreasing reactivity toward hydrolysis (most reactive on left): 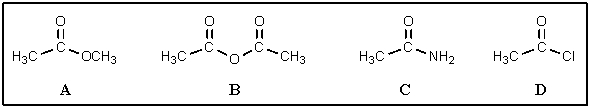
A) D > B > A > C
B) C > A > B > D
C) B > D > A > C
D) A > B > D > C
E) D > B > C > A

A) D > B > A > C
B) C > A > B > D
C) B > D > A > C
D) A > B > D > C
E) D > B > C > A

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
5
What would be the major expected product from the reaction shown below? 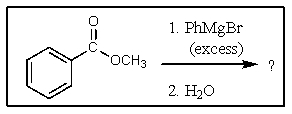
A)
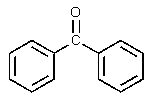
B)
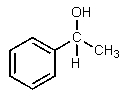
C)
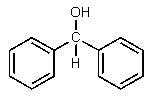
D)
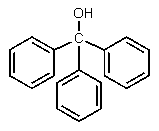
E)
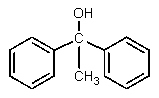

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the reactions listed below would produce benzyl acetate (= benzyl ethanoate)?
A)
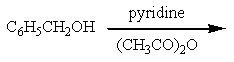
B)
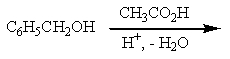
C)
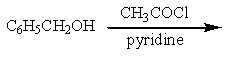
D) two of these
E) all of these
A)
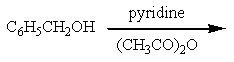
B)
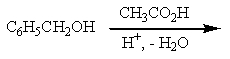
C)
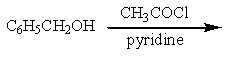
D) two of these
E) all of these

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
7
What type of functional group do the natural products known as waxes have?
A) Ester
B) Amide
C) Carboxylic acid
D) Alcohol
E) Amine
A) Ester
B) Amide
C) Carboxylic acid
D) Alcohol
E) Amine

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
8
What would be the organic product of the following reaction? 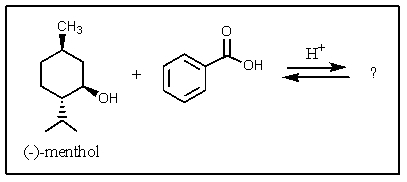
A)
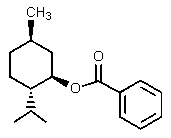
B)
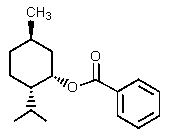
C) mixture of A and B
D) None of these.
E) No reaction occurs.

A)

B)

C) mixture of A and B
D) None of these.
E) No reaction occurs.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
9
What would be the name of the following cyclic ester? 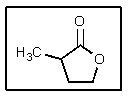
A) (-valerolactone)
B) (-butyrolactone)
C) 2-methyl--butyrolactone
D) 2-methyl--butyrolactone
E) 2-methyl--valerolactone

A) (-valerolactone)
B) (-butyrolactone)
C) 2-methyl--butyrolactone
D) 2-methyl--butyrolactone
E) 2-methyl--valerolactone

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is not an intermediate in the Hofmann rearrangement of ethanamide to methylamine? 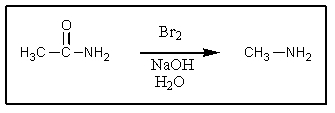
A)
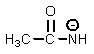
B)
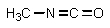
C)
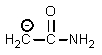
D)
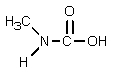
E)
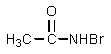

A)
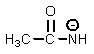
B)
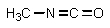
C)
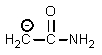
D)

E)
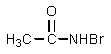

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
11
Lactones are:
A) Cyclic amides
B) Cyclic anhydrides
C) Cyclic esters
D) Cyclic acids
E) Cyclic ketones
A) Cyclic amides
B) Cyclic anhydrides
C) Cyclic esters
D) Cyclic acids
E) Cyclic ketones

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
12
Which reagent(s)would accomplish the following transformation? 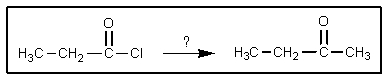
A) CH3Li
B) CH3I,NaOH
C) CH3MgBr
D) (CH3)2CuLi
E) either A or C

A) CH3Li
B) CH3I,NaOH
C) CH3MgBr
D) (CH3)2CuLi
E) either A or C

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
13
In the reaction shown below,which product(s)would be formed? 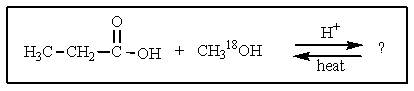
A)
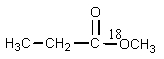
B)
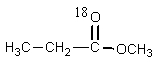
C)
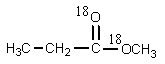
D)
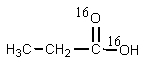
E) both A and B

A)
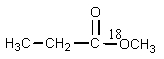
B)
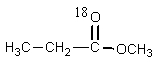
C)
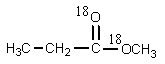
D)
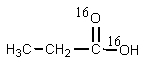
E) both A and B

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
14
How many different CH3 signals would you expect in the room-temperature proton NMR spectrum of the molecule below? 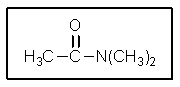
A) One
B) Two
C) Three
D) Four
E) Five

A) One
B) Two
C) Three
D) Four
E) Five

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
15
What would be the proper name of the following? 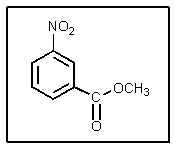
A) 3-nitro-methylbenzoate
B) methyl 3-nitrobenzoate
C) 3-nitro-methoxybenzoate
D) 5-nitro-methylbenzoate
E) 3-nitrocarboxymethylbenzene

A) 3-nitro-methylbenzoate
B) methyl 3-nitrobenzoate
C) 3-nitro-methoxybenzoate
D) 5-nitro-methylbenzoate
E) 3-nitrocarboxymethylbenzene

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
16
What would be the product of the following reaction? 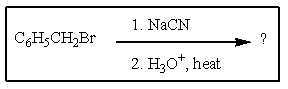
A) C6H5CO2H
B) C6H5CH2CO2H
C) C6H5CH2OH
D) C6H5CH2CH2NH2
E) C6H5CH3
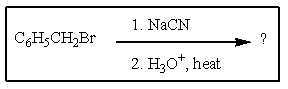
A) C6H5CO2H
B) C6H5CH2CO2H
C) C6H5CH2OH
D) C6H5CH2CH2NH2
E) C6H5CH3

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following would be properly named as 2-chloroethyl benzoate?
A)
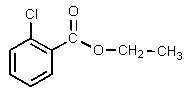
B)
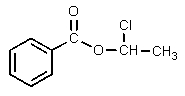
C)
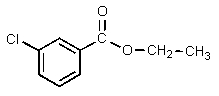
D)
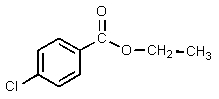
E)
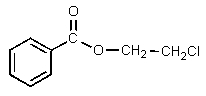
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
18
What would be the major organic product expected from the following reaction? 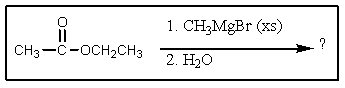
A)
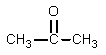
B)
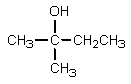
C)
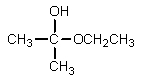
D)
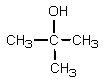
E)
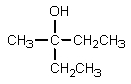

A)
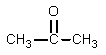
B)

C)

D)

E)


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
19
The product of the following reaction would be: 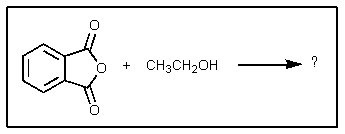
A)
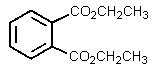
B)
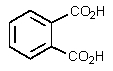
C)
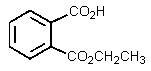
D)
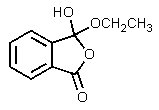
E)
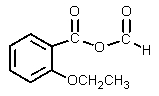

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
20
What would be the organic product of the following reaction? (18O is a rare oxygen isotope.) 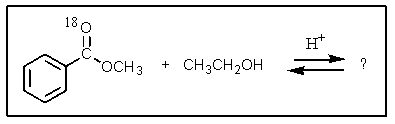
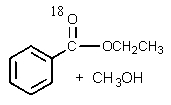
A)
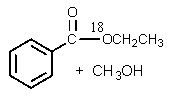
B)
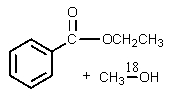
C)
D) More than one of these.
E) None of these.
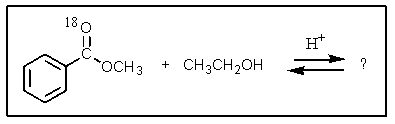
A)

B)

C)

D) More than one of these.
E) None of these.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
21
What reagent(s)would be required to achieve the following conversion? 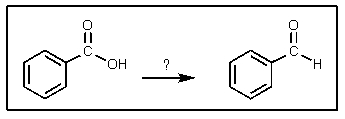
A)
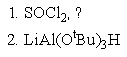
B)
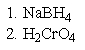
C)
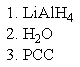
D)
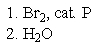
E) both A and C

A)
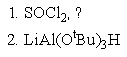
B)
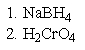
C)

D)
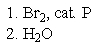
E) both A and C

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
22
How can the importance of the following resonance be evaluated? 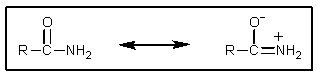
A) Charged resonance structures are always more important.
B) The C=O IR stretch for amides is significantly different than for other C=O groups.
C) The water solubility of amides implicates charged structures.
D) The C-N rotational barrier can be determined by NMR.
E) More than one of the above are correct.

A) Charged resonance structures are always more important.
B) The C=O IR stretch for amides is significantly different than for other C=O groups.
C) The water solubility of amides implicates charged structures.
D) The C-N rotational barrier can be determined by NMR.
E) More than one of the above are correct.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
23
Predict the product of the following reaction: 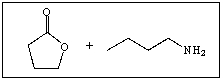
A)
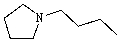
B)
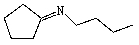
C)
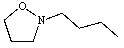
D)
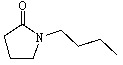
E) None of these.

A)

B)
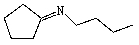
C)
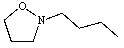
D)

E) None of these.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
24
Predict the product of the following reaction: 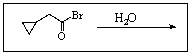
A)
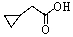
B)
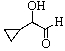
C)
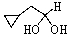
D)
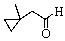
E) no reaction occurs

A)

B)

C)
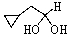
D)
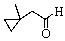
E) no reaction occurs

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
25
Predict the product of the following reaction: 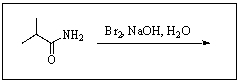
A)
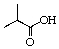
B)
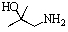
C)
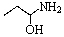
D)
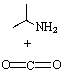
E)
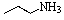

A)

B)
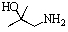
C)
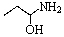
D)

E)
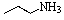

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following would be most likely to result from saponification of a natural triglyceride? 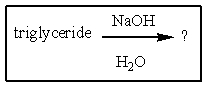
A)
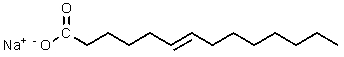
B)
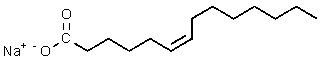
C)
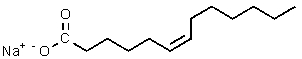
D)
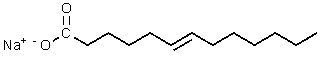
E)
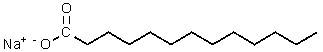
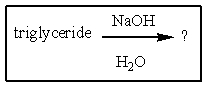
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
27
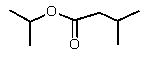
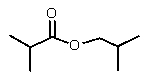
Reduction of which of the following esters with lithium aluminum hydride would yield two molecules of the same alcohol?
A)
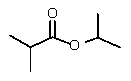
B)
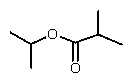
C)
D)
E) None of these.
A)

B)

C)

D)

E) None of these.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
28
A multistep preparation of propylpropanoate from only 1-propanol would require the use of how many of the reagents below (i.e.,what reagents would you need to use if you only had 1-propanol to start with)? 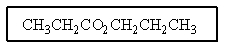
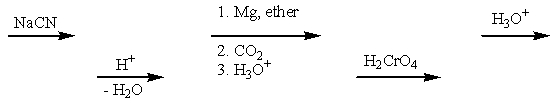
A) Two of the above.
B) Three of the above.
C) Four of the above.
D) All of the above.
E) The preparation cannot be done with only these reagents.
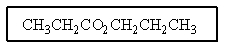
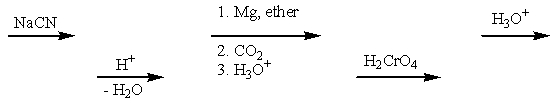
A) Two of the above.
B) Three of the above.
C) Four of the above.
D) All of the above.
E) The preparation cannot be done with only these reagents.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
29
When compound X is heated with aqueous acid,acetic acid and acetaldehyde (ethanal)are formed.The proton NMR spectrum of X is shown below.What is the structure of X? 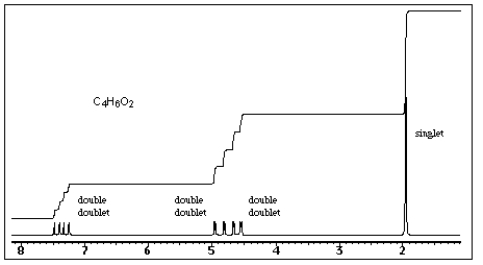
A) CH2=CHO2CCH3
B) CH2=CHOCH2CH3
C) CH2=C(CH3)O2CH
D) CH3CH=CHO2CH
E) CH2=CHCH2CO2H

A) CH2=CHO2CCH3
B) CH2=CHOCH2CH3
C) CH2=C(CH3)O2CH
D) CH3CH=CHO2CH
E) CH2=CHCH2CO2H

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
30
What is the product of combining pentanoic acid with thionyl chloride,followed by propanol and base?
A) pentanol
B) pentyl propanoate
C) propyl pentanoate
D) 1-tosyl pentanol
E) 1-chloropentanol
A) pentanol
B) pentyl propanoate
C) propyl pentanoate
D) 1-tosyl pentanol
E) 1-chloropentanol

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
31
What would be the other product of the following reaction? 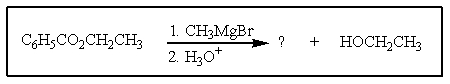
A)
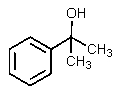
B)
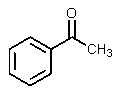
C)
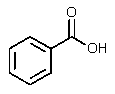
D)
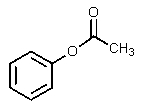
E)
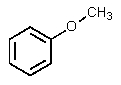
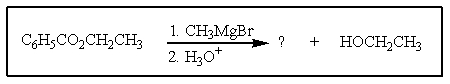
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
32
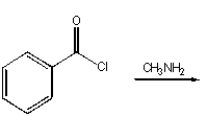
Which of the following reactions will produce N-methyl benzamide?
A)
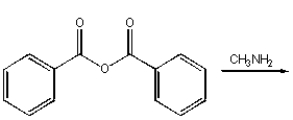
B)
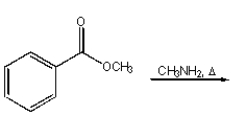
C)
D) both A and C
E) All of the above.
A)

B)

C)

D) both A and C
E) All of the above.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following is a lactam?
A)
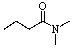
B)
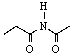
C)
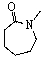
D)
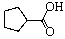
E) Two of these can be considered lactams.
A)

B)

C)
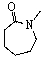
D)

E) Two of these can be considered lactams.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
34
Substitutions at sp2 carbons do not occur by an SN2 mechanism,but rather by way of a "tetrahedral intermediate." The tetrahedral intermediate shown could occur in which reaction? 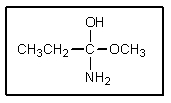
A) methyl propanoate + ammonia
B) propanamide + hydroxide
C) propanoic acid + methanol
D) propanamide + ethanol
E) None of these.

A) methyl propanoate + ammonia
B) propanamide + hydroxide
C) propanoic acid + methanol
D) propanamide + ethanol
E) None of these.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
35
What is the relative reactivity of carboxylic acid derivatives toward hydrolysis (left = least reactive)?
A) ester < amide < acid chloride < anhydride
B) amide < ester < acid chloride < anhydride
C) ester < amide < anhydride < acid chloride
D) amide < ester < anhydride < acid chloride
E) The relative reactivities really cannot be compared.
A) ester < amide < acid chloride < anhydride
B) amide < ester < acid chloride < anhydride
C) ester < amide < anhydride < acid chloride
D) amide < ester < anhydride < acid chloride
E) The relative reactivities really cannot be compared.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
36
What structure is not an intermediate in the following reaction? 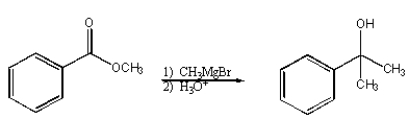
A)
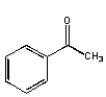
B)
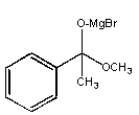
C)
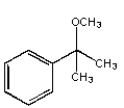
D)
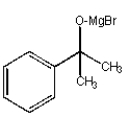
E) None of the above.
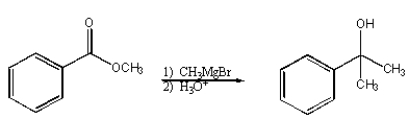
A)

B)

C)

D)

E) None of the above.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
37
Place the following compounds in order of increasing rate of hydrolysis. 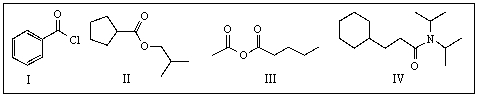
A) I,III,IV,II
B) IV,II,III,I
C) III,I,II,IV
D) I,II,IV,III
E) All carboxylic derivatives exhibit similar reactivities with H2O.

A) I,III,IV,II
B) IV,II,III,I
C) III,I,II,IV
D) I,II,IV,III
E) All carboxylic derivatives exhibit similar reactivities with H2O.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following reactions will not give a carboxylic acid as a product?
A)
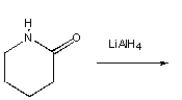
B)
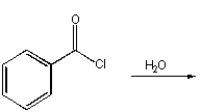
C)
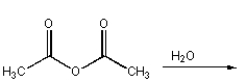
D)
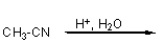
E)
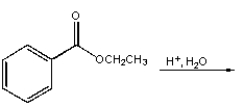
A)

B)

C)

D)
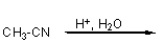
E)


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
39
What would be the products of the following reaction? 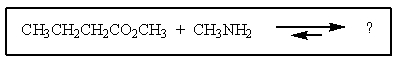
A) CH3CH2CH2CH2NH(CH3)+ H2O
B) CH3NHC(=O)CH2CH2CH3 + CH3OH
C) CH3CH2CH2CONH2 + CH3CH2OH
D) CH3OH + CH3CH2CH2OC(=O)NHCH3
E) CH3CH2CH2NH(CH3)COCH3 + H2O
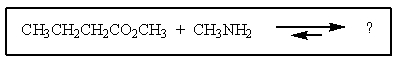
A) CH3CH2CH2CH2NH(CH3)+ H2O
B) CH3NHC(=O)CH2CH2CH3 + CH3OH
C) CH3CH2CH2CONH2 + CH3CH2OH
D) CH3OH + CH3CH2CH2OC(=O)NHCH3
E) CH3CH2CH2NH(CH3)COCH3 + H2O

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
40
What would be the product of the following reaction? 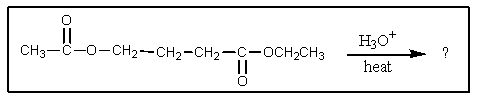
A)
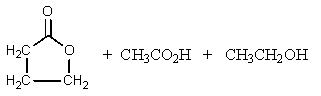
B)
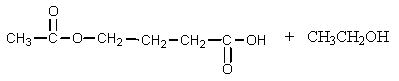
C)
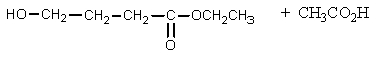
D)
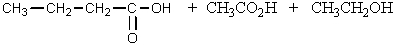
E) None of these.

A)
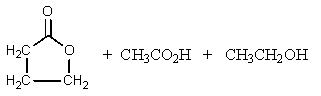
B)
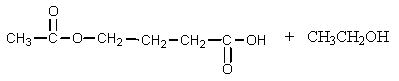
C)
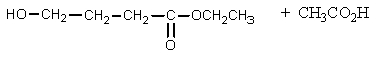
D)
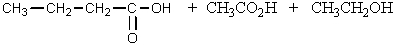
E) None of these.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
41
Which,if any,functional group in the molecule below would undergo basic hydrolysis most easily? 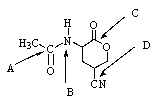
A) A
B) B
C) C
D) D
E) None of these groups would react.

A) A
B) B
C) C
D) D
E) None of these groups would react.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
42
What reagent is needed to complete the reaction shown? 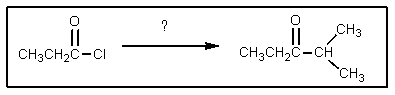
A) ((CH3)2CH)2CuLi
B) KCN/NaOH
C) HOCH(CH3)2
D) BrMgCH(CH3)2
E) (CH3)2CHLi

A) ((CH3)2CH)2CuLi
B) KCN/NaOH
C) HOCH(CH3)2
D) BrMgCH(CH3)2
E) (CH3)2CHLi

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
43
Which functional group has the lowest IR absorption frequency?
A)
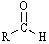
B)
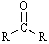
C)
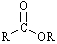
D)
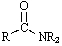
E)
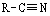
A)
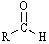
B)
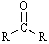
C)
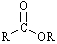
D)
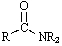
E)

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
44
What is the product from the following reaction? 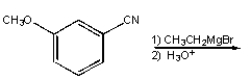
A)
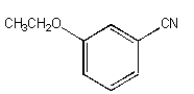
B)
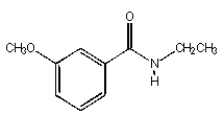
C)
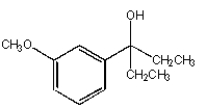
D)
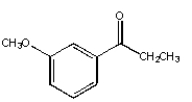
E)
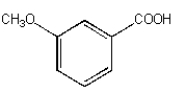

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck


