Deck 19: Carboxylic Acids
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/27
Play
Full screen (f)
Deck 19: Carboxylic Acids
1
Which of the following sets of reagents would accomplish the chemical transformation shown? 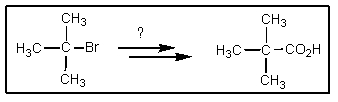
A)
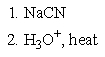
B)
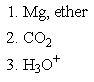
C)
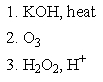
D)
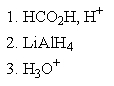
E)
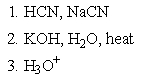

A)
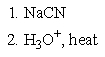
B)
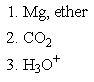
C)
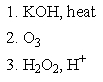
D)
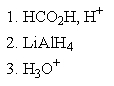
E)
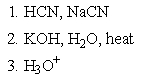
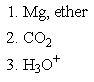
2
What would be the major organic product of the following reaction? 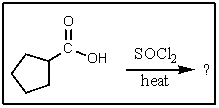
A)
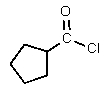
B)
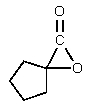
C)
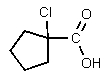
D)
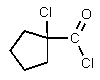
E)
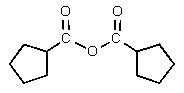

A)

B)

C)

D)

E)


3
Which would be the best name of the following compound? 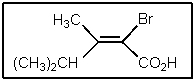
A) 2-bromo-3-isopropylbutenoic acid
B) (E)-2-bromo-3,4-dimethyl-2-pentenoic acid
C) (E)-2-bromo-3-methyl-2-hexenoic acid
D) (E)-2-bromo-3-methyl-2-pentenoic acid
E) (Z)-2-bromo-3-methyl-2-pentenoic acid

A) 2-bromo-3-isopropylbutenoic acid
B) (E)-2-bromo-3,4-dimethyl-2-pentenoic acid
C) (E)-2-bromo-3-methyl-2-hexenoic acid
D) (E)-2-bromo-3-methyl-2-pentenoic acid
E) (Z)-2-bromo-3-methyl-2-pentenoic acid
(E)-2-bromo-3,4-dimethyl-2-pentenoic acid
4
What best accounts for the unusually high boiling points of carboxylic acids relative to other organic compounds of similar molecular weight?
A) The relatively large concentration of oxygen atoms.
B) The presence of intramolecular hydrogen bonding.
C) Carboxylic acids are mostly ionized and thus are mostly ionic compounds.
D) The presence of intermolecular hydrogen bonding.
E) Carboxylic acids do not have unusually high boiling points.
A) The relatively large concentration of oxygen atoms.
B) The presence of intramolecular hydrogen bonding.
C) Carboxylic acids are mostly ionized and thus are mostly ionic compounds.
D) The presence of intermolecular hydrogen bonding.
E) Carboxylic acids do not have unusually high boiling points.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
5
What major product would result from the following reaction? 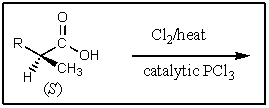
A)
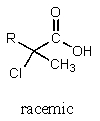
B)
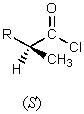
C)
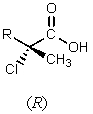
D)
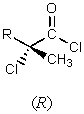
E)
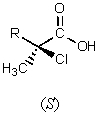

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
6
What reagent(s)is (are)needed to complete the reaction shown? 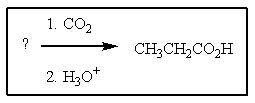
A) CH3CH2Br
B) KMnO4/ -OH
C) CH3CH2MgBr
D) CH3Li
E) CH3COCl

A) CH3CH2Br
B) KMnO4/ -OH
C) CH3CH2MgBr
D) CH3Li
E) CH3COCl

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
7
Rank the following in decreasing order of acidity (most acidic on left): 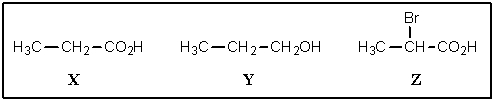
A) X > Z > Y
B) Y > X > Z
C) Z > X > Y
D) Z > Y > X
E) X > Y > Z

A) X > Z > Y
B) Y > X > Z
C) Z > X > Y
D) Z > Y > X
E) X > Y > Z

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
8
Which,if any,of the reactions below would produce 2-methylpropanoic acid?
A)
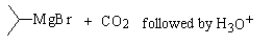
B)
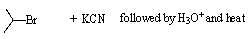
C)
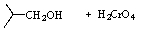
D) All of these reactions would produce the desired product.
E) None of these reactions would produce the desired product.
A)
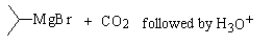
B)
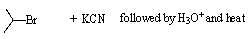
C)
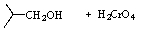
D) All of these reactions would produce the desired product.
E) None of these reactions would produce the desired product.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
9
What reagent(s)would accomplish the following conversion? 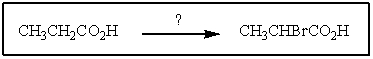
A) Br2
B) PBr3
C) Br2/light
D) Br2 + cat.PBr3
E) PBr3 + cat.Br2

A) Br2
B) PBr3
C) Br2/light
D) Br2 + cat.PBr3
E) PBr3 + cat.Br2

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
10
The reaction of propanoic acid with lithium aluminum hydride,followed by water,would result in what product?
A)
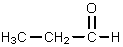
B)
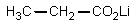
C)
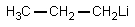
D)
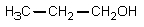
E)
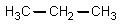
A)
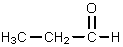
B)
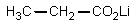
C)
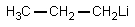
D)
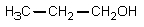
E)
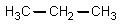

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
11
What would the common name of the following di-acid be? 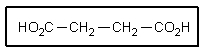
A) Malonic acid
B) Oxalic acid
C) Succinic acid
D) Adipic acid
E) Glutaric acid
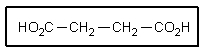
A) Malonic acid
B) Oxalic acid
C) Succinic acid
D) Adipic acid
E) Glutaric acid

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
12
Which would be the strongest acid of the following?
A) BrCH2CH2CO2H
B) ClCH2CH2CO2H
C) CH3CHClCO2H
D) CF3CO2H
E) CH3CHClCOCl
A) BrCH2CH2CO2H
B) ClCH2CH2CO2H
C) CH3CHClCO2H
D) CF3CO2H
E) CH3CHClCOCl

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
13
The higher acidity of carboxylic acids compared to other functional groups is best explained by
A) hydrogen bonding.
B) electronegativity of oxygen.
C) water solubility.
D) resonance.
E) electronegativity of carbon.
A) hydrogen bonding.
B) electronegativity of oxygen.
C) water solubility.
D) resonance.
E) electronegativity of carbon.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following sets of reagents would convert benzyl bromide to phenylethanoic acid? 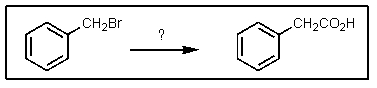
A)
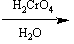
B)
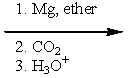
C)
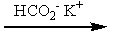
D)
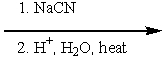
E) both B and D

A)
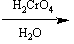
B)
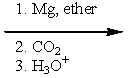
C)
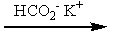
D)
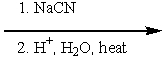
E) both B and D

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
15
What would be the organic product of the following reaction? 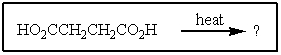
A) CH3CH2CO2H + CO2
B) 2 CH3CO2H
C)
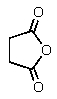
D)
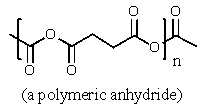
E) None of these are formed.

A) CH3CH2CO2H + CO2
B) 2 CH3CO2H
C)

D)

E) None of these are formed.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
16
Several 2-arylpropanoic acids are used as analgesics (painkillers).Three of these [Ibuprofen (= Advil),Naproxen (= Aleve)and Ketoprofen (= Orudis KT)] are currently available in over-the-counter form.Which of the following sets of reagents would accomplish conversion of an ethyl aromatic to a 2-arylpropanoic acid? ![<strong>Several 2-arylpropanoic acids are used as analgesics (painkillers).Three of these [Ibuprofen (= Advil),Naproxen (= Aleve)and Ketoprofen (= Orudis KT)] are currently available in over-the-counter form.Which of the following sets of reagents would accomplish conversion of an ethyl aromatic to a 2-arylpropanoic acid? </strong> A) B) C) D) E) both A and B](https://storage.examlex.com/TB6928/11eab5f7_3d94_4d15_a628_3fd335664c0c_TB6928_00.jpg)
A)
![<strong>Several 2-arylpropanoic acids are used as analgesics (painkillers).Three of these [Ibuprofen (= Advil),Naproxen (= Aleve)and Ketoprofen (= Orudis KT)] are currently available in over-the-counter form.Which of the following sets of reagents would accomplish conversion of an ethyl aromatic to a 2-arylpropanoic acid? </strong> A) B) C) D) E) both A and B](https://storage.examlex.com/TB6928/11eab5f7_3d94_4d16_a628_e1e84caa24eb_TB6928_00.jpg)
B)
![<strong>Several 2-arylpropanoic acids are used as analgesics (painkillers).Three of these [Ibuprofen (= Advil),Naproxen (= Aleve)and Ketoprofen (= Orudis KT)] are currently available in over-the-counter form.Which of the following sets of reagents would accomplish conversion of an ethyl aromatic to a 2-arylpropanoic acid? </strong> A) B) C) D) E) both A and B](https://storage.examlex.com/TB6928/11eab5f7_3d94_7427_a628_e19baf5ea32d_TB6928_00.jpg)
C)
![<strong>Several 2-arylpropanoic acids are used as analgesics (painkillers).Three of these [Ibuprofen (= Advil),Naproxen (= Aleve)and Ketoprofen (= Orudis KT)] are currently available in over-the-counter form.Which of the following sets of reagents would accomplish conversion of an ethyl aromatic to a 2-arylpropanoic acid? </strong> A) B) C) D) E) both A and B](https://storage.examlex.com/TB6928/11eab5f7_3d94_7428_a628_bf50c8262ab7_TB6928_00.jpg)
D)
![<strong>Several 2-arylpropanoic acids are used as analgesics (painkillers).Three of these [Ibuprofen (= Advil),Naproxen (= Aleve)and Ketoprofen (= Orudis KT)] are currently available in over-the-counter form.Which of the following sets of reagents would accomplish conversion of an ethyl aromatic to a 2-arylpropanoic acid? </strong> A) B) C) D) E) both A and B](https://storage.examlex.com/TB6928/11eab5f7_3d94_7429_a628_6ba5013356ea_TB6928_00.jpg)
E) both A and B
![<strong>Several 2-arylpropanoic acids are used as analgesics (painkillers).Three of these [Ibuprofen (= Advil),Naproxen (= Aleve)and Ketoprofen (= Orudis KT)] are currently available in over-the-counter form.Which of the following sets of reagents would accomplish conversion of an ethyl aromatic to a 2-arylpropanoic acid? </strong> A) B) C) D) E) both A and B](https://storage.examlex.com/TB6928/11eab5f7_3d94_4d15_a628_3fd335664c0c_TB6928_00.jpg)
A)
![<strong>Several 2-arylpropanoic acids are used as analgesics (painkillers).Three of these [Ibuprofen (= Advil),Naproxen (= Aleve)and Ketoprofen (= Orudis KT)] are currently available in over-the-counter form.Which of the following sets of reagents would accomplish conversion of an ethyl aromatic to a 2-arylpropanoic acid? </strong> A) B) C) D) E) both A and B](https://storage.examlex.com/TB6928/11eab5f7_3d94_4d16_a628_e1e84caa24eb_TB6928_00.jpg)
B)
![<strong>Several 2-arylpropanoic acids are used as analgesics (painkillers).Three of these [Ibuprofen (= Advil),Naproxen (= Aleve)and Ketoprofen (= Orudis KT)] are currently available in over-the-counter form.Which of the following sets of reagents would accomplish conversion of an ethyl aromatic to a 2-arylpropanoic acid? </strong> A) B) C) D) E) both A and B](https://storage.examlex.com/TB6928/11eab5f7_3d94_7427_a628_e19baf5ea32d_TB6928_00.jpg)
C)
![<strong>Several 2-arylpropanoic acids are used as analgesics (painkillers).Three of these [Ibuprofen (= Advil),Naproxen (= Aleve)and Ketoprofen (= Orudis KT)] are currently available in over-the-counter form.Which of the following sets of reagents would accomplish conversion of an ethyl aromatic to a 2-arylpropanoic acid? </strong> A) B) C) D) E) both A and B](https://storage.examlex.com/TB6928/11eab5f7_3d94_7428_a628_bf50c8262ab7_TB6928_00.jpg)
D)
![<strong>Several 2-arylpropanoic acids are used as analgesics (painkillers).Three of these [Ibuprofen (= Advil),Naproxen (= Aleve)and Ketoprofen (= Orudis KT)] are currently available in over-the-counter form.Which of the following sets of reagents would accomplish conversion of an ethyl aromatic to a 2-arylpropanoic acid? </strong> A) B) C) D) E) both A and B](https://storage.examlex.com/TB6928/11eab5f7_3d94_7429_a628_6ba5013356ea_TB6928_00.jpg)
E) both A and B

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
17
What would be the product of the following reaction? 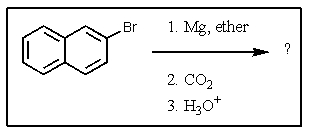
A)
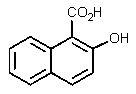
B)
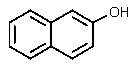
C)
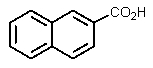
D)
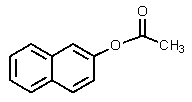
E) none of these

A)

B)

C)

D)

E) none of these

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
18
What reagent is needed to complete the reaction shown? 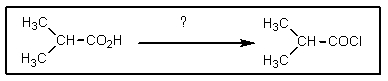
A) CH3Cl
B) PCl3
C) Cl2
D) LiAlH4
E) none of these

A) CH3Cl
B) PCl3
C) Cl2
D) LiAlH4
E) none of these

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
19
What reagent(s)would accomplish the following? 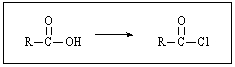
A) Cl2,light
B) Cl2,H2O
C) SOCl2
D) MgCl2
E) aqueous HCl

A) Cl2,light
B) Cl2,H2O
C) SOCl2
D) MgCl2
E) aqueous HCl

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
20
What would be the name of the following? 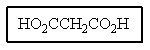
A) Malonic acid
B) Propanoic acid
C) Oxalic acid
D) Succinic acid
E) Butanedioic acid
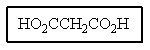
A) Malonic acid
B) Propanoic acid
C) Oxalic acid
D) Succinic acid
E) Butanedioic acid

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
21
Rank the following in order of decreasing acidity (more acidic > less acidic): 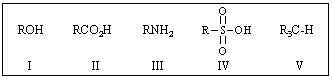
A) II > III > I > IV > V
B) IV > II > I > III > V
C) II > IV > I > III > V
D) V > III > I > II > IV
E) II > IV > I > V > III

A) II > III > I > IV > V
B) IV > II > I > III > V
C) II > IV > I > III > V
D) V > III > I > II > IV
E) II > IV > I > V > III

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
22
Carboxylic acids have unusually high boiling points because:
A) they are significantly heavier than organic molecules with the same molecular weight.
B) they repel each other in the gas phase.
C) they strongly attract each other in the liquid phase.
D) they are usually solids,and solids are not volatile.
E) they exist mostly as ions (RCO2- + H+)and ionic materials are not volatile.
A) they are significantly heavier than organic molecules with the same molecular weight.
B) they repel each other in the gas phase.
C) they strongly attract each other in the liquid phase.
D) they are usually solids,and solids are not volatile.
E) they exist mostly as ions (RCO2- + H+)and ionic materials are not volatile.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
23
Rank the following benzoic acids in order of decreasing acidity: 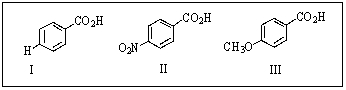
A) III > I > II
B) I > III > II
C) II > I > III
D) I > II > III
E) III > II > I

A) III > I > II
B) I > III > II
C) II > I > III
D) I > II > III
E) III > II > I

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
24
In the carboxylic acid dimer (below),which bond(s)has a strength of about 6-8 kcal/mol? 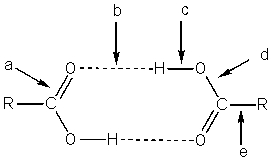
A) Bond a
B) Bond b
C) Bond c
D) Bond d
E) Bond e

A) Bond a
B) Bond b
C) Bond c
D) Bond d
E) Bond e

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following intermediates are not involved in the acid catalyzed esterification reaction of a carboxylic acid?
A)
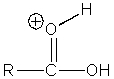
B)
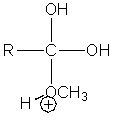
C)
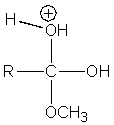
D)
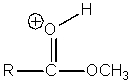
E) All of the above are intermediates.
A)

B)

C)

D)

E) All of the above are intermediates.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
26
Which compound best fits the following spectroscopic data? 1H NMR = 1.00 (t,J=7.4 Hz,3H); 1.65 (sextet,J=7.5 Hz,2H); 2.31 (t,J=7.4 Hz,2H); and 11.68 (s,1H)ppm.13C NMR = 13.4,18.5,36.3,179.6 ppm.
A)
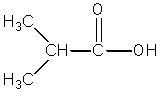
B)
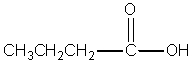
C)
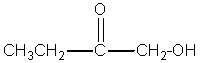
D)
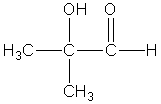
E) none of the above
A)

B)
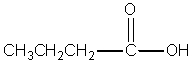
C)

D)

E) none of the above

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
27
What is the IUPAC name of the following compound? 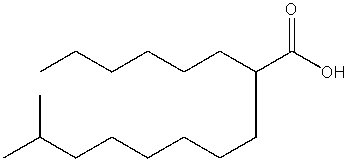
A) 2-hexyl-9-methyldecanoic acid
B) 2-isononyloctanoic acid
C) 1-methyl-9-carboxypentadecane
D) 2-hexyldecanoic acid
E) 14-methyl-7-carboxypentadecane

A) 2-hexyl-9-methyldecanoic acid
B) 2-isononyloctanoic acid
C) 1-methyl-9-carboxypentadecane
D) 2-hexyldecanoic acid
E) 14-methyl-7-carboxypentadecane

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck


