Deck 2: Polar Covalent Bonds;acids and Bases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/50
Play
Full screen (f)
Deck 2: Polar Covalent Bonds;acids and Bases
1
Exhibit 2-2
Calculate the formal charges on the indicated atoms in each compound below.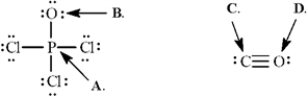
Refer to Exhibit 2-2.The formal charge on oxygen (B) is ______.
Calculate the formal charges on the indicated atoms in each compound below.
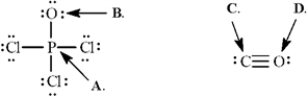
Refer to Exhibit 2-2.The formal charge on oxygen (B) is ______.
−1
2
Exhibit 2-2
Calculate the formal charges on the indicated atoms in each compound below.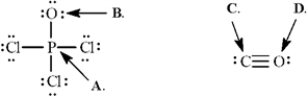
Refer to Exhibit 2-2.The formal charge on carbon (C) is ______.
Calculate the formal charges on the indicated atoms in each compound below.
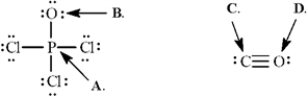
Refer to Exhibit 2-2.The formal charge on carbon (C) is ______.
−1
3
Exhibit 2-1
Give the corresponding letter of the term that best matches the given definition.
a.Brønsted-Lowry Acid
f.Ionic Bond
b.Brønsted-Lowry Base
g.Covalent Bond
c.Lewis Acid
h.Polar-Covalent Bond
d.Lewis Base
i.Hydrophobic
e.Electronegativity
j.Hydrophilic
_____ A compound that can donate a proton.
Give the corresponding letter of the term that best matches the given definition.
a.Brønsted-Lowry Acid
f.Ionic Bond
b.Brønsted-Lowry Base
g.Covalent Bond
c.Lewis Acid
h.Polar-Covalent Bond
d.Lewis Base
i.Hydrophobic
e.Electronegativity
j.Hydrophilic
_____ A compound that can donate a proton.
a
4
Exhibit 2-4
Use the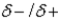 convention and the crossed arrow
convention and the crossed arrow  to show the direction of the expected polarity of the indicated bonds in the following compounds.
to show the direction of the expected polarity of the indicated bonds in the following compounds.
Refer to Exhibit 2-4.The C−O bond in furan,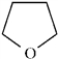
Use the
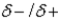 convention and the crossed arrow
convention and the crossed arrow  to show the direction of the expected polarity of the indicated bonds in the following compounds.
to show the direction of the expected polarity of the indicated bonds in the following compounds.Refer to Exhibit 2-4.The C−O bond in furan,


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
5
Exhibit 2-1
Give the corresponding letter of the term that best matches the given definition.
a.Brønsted-Lowry Acid
f.Ionic Bond
b.Brønsted-Lowry Base
g.Covalent Bond
c.Lewis Acid
h.Polar-Covalent Bond
d.Lewis Base
i.Hydrophobic
e.Electronegativity
j.Hydrophilic
_____ The ability of an atom to attract the shared electrons in a covalent bond.
Give the corresponding letter of the term that best matches the given definition.
a.Brønsted-Lowry Acid
f.Ionic Bond
b.Brønsted-Lowry Base
g.Covalent Bond
c.Lewis Acid
h.Polar-Covalent Bond
d.Lewis Base
i.Hydrophobic
e.Electronegativity
j.Hydrophilic
_____ The ability of an atom to attract the shared electrons in a covalent bond.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
6
Exhibit 2-2
Calculate the formal charges on the indicated atoms in each compound below.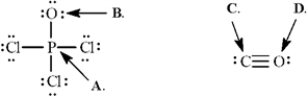
Refer to Exhibit 2-2.The formal charge on phosphorous (A) is ______.
Calculate the formal charges on the indicated atoms in each compound below.
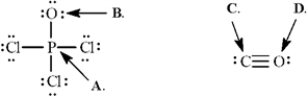
Refer to Exhibit 2-2.The formal charge on phosphorous (A) is ______.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
7
Exhibit 2-3
Phenylalanine is an amino acid that is essential to human nutrition.The representation below shows the structure of phenylalanine at physiological pH.Consider this structure to answer the following question(s).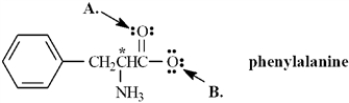
Refer to Exhibit 2-3.The oxygen atom labeled B.has _____ bonding electrons.
Phenylalanine is an amino acid that is essential to human nutrition.The representation below shows the structure of phenylalanine at physiological pH.Consider this structure to answer the following question(s).

Refer to Exhibit 2-3.The oxygen atom labeled B.has _____ bonding electrons.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
8
Exhibit 2-1
Give the corresponding letter of the term that best matches the given definition.
a.Brønsted-Lowry Acid
f.Ionic Bond
b.Brønsted-Lowry Base
g.Covalent Bond
c.Lewis Acid
h.Polar-Covalent Bond
d.Lewis Base
i.Hydrophobic
e.Electronegativity
j.Hydrophilic
_____ A bond between two atoms differing in electronegativity by > 2.
Give the corresponding letter of the term that best matches the given definition.
a.Brønsted-Lowry Acid
f.Ionic Bond
b.Brønsted-Lowry Base
g.Covalent Bond
c.Lewis Acid
h.Polar-Covalent Bond
d.Lewis Base
i.Hydrophobic
e.Electronegativity
j.Hydrophilic
_____ A bond between two atoms differing in electronegativity by > 2.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
9
Exhibit 2-1
Give the corresponding letter of the term that best matches the given definition.
a.Brønsted-Lowry Acid
f.Ionic Bond
b.Brønsted-Lowry Base
g.Covalent Bond
c.Lewis Acid
h.Polar-Covalent Bond
d.Lewis Base
i.Hydrophobic
e.Electronegativity
j.Hydrophilic
_____ A compound that can accept a proton.
Give the corresponding letter of the term that best matches the given definition.
a.Brønsted-Lowry Acid
f.Ionic Bond
b.Brønsted-Lowry Base
g.Covalent Bond
c.Lewis Acid
h.Polar-Covalent Bond
d.Lewis Base
i.Hydrophobic
e.Electronegativity
j.Hydrophilic
_____ A compound that can accept a proton.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
10
Exhibit 2-4
Use the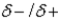 convention and the crossed arrow
convention and the crossed arrow 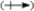 to show the direction of the expected polarity of the indicated bonds in the following compounds.
to show the direction of the expected polarity of the indicated bonds in the following compounds.
Refer to Exhibit 2-4.The C−Si bond in tetramethylsilane, (CH3)4Si
Use the
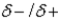 convention and the crossed arrow
convention and the crossed arrow 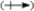 to show the direction of the expected polarity of the indicated bonds in the following compounds.
to show the direction of the expected polarity of the indicated bonds in the following compounds.Refer to Exhibit 2-4.The C−Si bond in tetramethylsilane, (CH3)4Si

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
11
Exhibit 2-1
Give the corresponding letter of the term that best matches the given definition.
a.Brønsted-Lowry Acid
f.Ionic Bond
b.Brønsted-Lowry Base
g.Covalent Bond
c.Lewis Acid
h.Polar-Covalent Bond
d.Lewis Base
i.Hydrophobic
e.Electronegativity
j.Hydrophilic
_____ A term used to describe a "water loving" species.
Give the corresponding letter of the term that best matches the given definition.
a.Brønsted-Lowry Acid
f.Ionic Bond
b.Brønsted-Lowry Base
g.Covalent Bond
c.Lewis Acid
h.Polar-Covalent Bond
d.Lewis Base
i.Hydrophobic
e.Electronegativity
j.Hydrophilic
_____ A term used to describe a "water loving" species.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
12
Exhibit 2-3
Phenylalanine is an amino acid that is essential to human nutrition.The representation below shows the structure of phenylalanine at physiological pH.Consider this structure to answer the following question(s).
Refer to Exhibit 2-3.Assign any formal charges to atoms in this representation of phenylalanine.
Phenylalanine is an amino acid that is essential to human nutrition.The representation below shows the structure of phenylalanine at physiological pH.Consider this structure to answer the following question(s).

Refer to Exhibit 2-3.Assign any formal charges to atoms in this representation of phenylalanine.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
13
Exhibit 2-1
Give the corresponding letter of the term that best matches the given definition.
a.Brønsted-Lowry Acid
f.Ionic Bond
b.Brønsted-Lowry Base
g.Covalent Bond
c.Lewis Acid
h.Polar-Covalent Bond
d.Lewis Base
i.Hydrophobic
e.Electronegativity
j.Hydrophilic
_____ Any species that accepts electrons.
Give the corresponding letter of the term that best matches the given definition.
a.Brønsted-Lowry Acid
f.Ionic Bond
b.Brønsted-Lowry Base
g.Covalent Bond
c.Lewis Acid
h.Polar-Covalent Bond
d.Lewis Base
i.Hydrophobic
e.Electronegativity
j.Hydrophilic
_____ Any species that accepts electrons.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
14
Exhibit 2-3
Phenylalanine is an amino acid that is essential to human nutrition.The representation below shows the structure of phenylalanine at physiological pH.Consider this structure to answer the following question(s).
Refer to Exhibit 2-3.The oxygen atom labeled A.has ______ non-bonding electrons.
Phenylalanine is an amino acid that is essential to human nutrition.The representation below shows the structure of phenylalanine at physiological pH.Consider this structure to answer the following question(s).

Refer to Exhibit 2-3.The oxygen atom labeled A.has ______ non-bonding electrons.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
15
Exhibit 2-4
Use the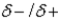 convention and the crossed arrow
convention and the crossed arrow  to show the direction of the expected polarity of the indicated bonds in the following compounds.
to show the direction of the expected polarity of the indicated bonds in the following compounds.
Refer to Exhibit 2-4.The C−F bond in fluorobenzene,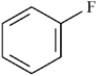
Use the
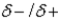 convention and the crossed arrow
convention and the crossed arrow  to show the direction of the expected polarity of the indicated bonds in the following compounds.
to show the direction of the expected polarity of the indicated bonds in the following compounds.Refer to Exhibit 2-4.The C−F bond in fluorobenzene,


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
16
Exhibit 2-1
Give the corresponding letter of the term that best matches the given definition.
a.Brønsted-Lowry Acid
f.Ionic Bond
b.Brønsted-Lowry Base
g.Covalent Bond
c.Lewis Acid
h.Polar-Covalent Bond
d.Lewis Base
i.Hydrophobic
e.Electronegativity
j.Hydrophilic
_____ A bond between two atoms differing in electronegativity by < 0.5.
Give the corresponding letter of the term that best matches the given definition.
a.Brønsted-Lowry Acid
f.Ionic Bond
b.Brønsted-Lowry Base
g.Covalent Bond
c.Lewis Acid
h.Polar-Covalent Bond
d.Lewis Base
i.Hydrophobic
e.Electronegativity
j.Hydrophilic
_____ A bond between two atoms differing in electronegativity by < 0.5.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
17
Exhibit 2-1
Give the corresponding letter of the term that best matches the given definition.
a.Brønsted-Lowry Acid
f.Ionic Bond
b.Brønsted-Lowry Base
g.Covalent Bond
c.Lewis Acid
h.Polar-Covalent Bond
d.Lewis Base
i.Hydrophobic
e.Electronegativity
j.Hydrophilic
_____ A bond between two atoms differing in electronegativity by 0.5 − 2.
Give the corresponding letter of the term that best matches the given definition.
a.Brønsted-Lowry Acid
f.Ionic Bond
b.Brønsted-Lowry Base
g.Covalent Bond
c.Lewis Acid
h.Polar-Covalent Bond
d.Lewis Base
i.Hydrophobic
e.Electronegativity
j.Hydrophilic
_____ A bond between two atoms differing in electronegativity by 0.5 − 2.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
18
Exhibit 2-2
Calculate the formal charges on the indicated atoms in each compound below.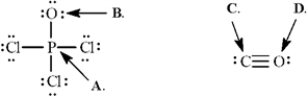
Refer to Exhibit 2-2.The formal charge on oxygen (D) is ______.
Calculate the formal charges on the indicated atoms in each compound below.
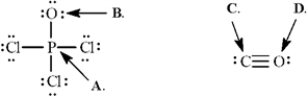
Refer to Exhibit 2-2.The formal charge on oxygen (D) is ______.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
19
Exhibit 2-1
Give the corresponding letter of the term that best matches the given definition.
a.Brønsted-Lowry Acid
f.Ionic Bond
b.Brønsted-Lowry Base
g.Covalent Bond
c.Lewis Acid
h.Polar-Covalent Bond
d.Lewis Base
i.Hydrophobic
e.Electronegativity
j.Hydrophilic
_____ A term used to describe a "water fearing" species.
Give the corresponding letter of the term that best matches the given definition.
a.Brønsted-Lowry Acid
f.Ionic Bond
b.Brønsted-Lowry Base
g.Covalent Bond
c.Lewis Acid
h.Polar-Covalent Bond
d.Lewis Base
i.Hydrophobic
e.Electronegativity
j.Hydrophilic
_____ A term used to describe a "water fearing" species.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
20
Exhibit 2-1
Give the corresponding letter of the term that best matches the given definition.
a.Brønsted-Lowry Acid
f.Ionic Bond
b.Brønsted-Lowry Base
g.Covalent Bond
c.Lewis Acid
h.Polar-Covalent Bond
d.Lewis Base
i.Hydrophobic
e.Electronegativity
j.Hydrophilic
_____ Any species that donates electrons.
Give the corresponding letter of the term that best matches the given definition.
a.Brønsted-Lowry Acid
f.Ionic Bond
b.Brønsted-Lowry Base
g.Covalent Bond
c.Lewis Acid
h.Polar-Covalent Bond
d.Lewis Base
i.Hydrophobic
e.Electronegativity
j.Hydrophilic
_____ Any species that donates electrons.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
21
Draw two resonance structures for the species below.
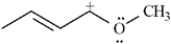


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
22
An acid with a low pKa:
A)is a weak acid
B)is a strong acid
C)has a weak conjugate base
D)both b and c
A)is a weak acid
B)is a strong acid
C)has a weak conjugate base
D)both b and c

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
23
Draw two resonance structures for the species below. 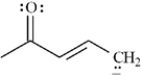


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
24
Exhibit 2-8
Consider the reaction below to answer the following question(s).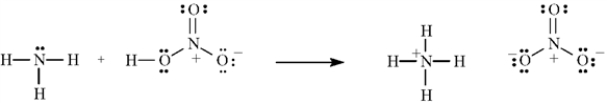
Refer to Exhibit 2-8.Using the curved arrow formalism,show the flow of electrons for this reaction.
Consider the reaction below to answer the following question(s).

Refer to Exhibit 2-8.Using the curved arrow formalism,show the flow of electrons for this reaction.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
25
The following is a representation of the pain reliever,acetaminophen,the active ingredient in Tylenol .Indicate the positions of any multiple bonds.Atoms other than carbon and hydrogen are labeled. 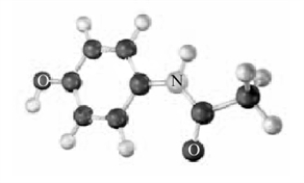


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
26
Circle all the Lewis bases in the group of compounds below. 


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
27
Exhibit 2-10
Consider the acid-base reaction below to answer the following question(s).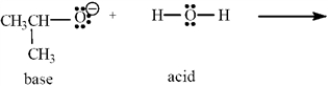
Refer to Exhibit 2-10.Using the curved arrow formalism,show the flow of electrons for this reaction.
Consider the acid-base reaction below to answer the following question(s).
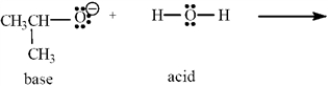
Refer to Exhibit 2-10.Using the curved arrow formalism,show the flow of electrons for this reaction.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
28
Exhibit 2-9
Indole is pleasant smelling in highly dilute solutions and has been used in perfumery.Use the structure of indole,below,to answer the following question(s).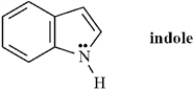
Refer to Exhibit 2-9.Indole can function as a Brønsted-Lowry acid in the presence of strong bases.Formulate a reaction,showing electron flow with arrows,that demonstrates this reactivity of indole.
Indole is pleasant smelling in highly dilute solutions and has been used in perfumery.Use the structure of indole,below,to answer the following question(s).

Refer to Exhibit 2-9.Indole can function as a Brønsted-Lowry acid in the presence of strong bases.Formulate a reaction,showing electron flow with arrows,that demonstrates this reactivity of indole.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
29
Exhibit 2-6
Refer to the following equation to answer the question(s) below.Place the letter corresponding to the correct answer in the blank.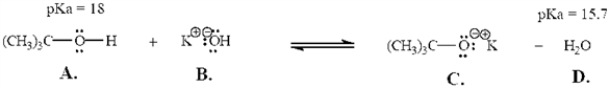
Refer to Exhibit 2-6.Will this reaction take place as written? Explain.
Refer to the following equation to answer the question(s) below.Place the letter corresponding to the correct answer in the blank.

Refer to Exhibit 2-6.Will this reaction take place as written? Explain.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
30
Exhibit 2-8
Consider the reaction below to answer the following question(s).
Refer to Exhibit 2-8.Label the acid and the base in the reaction.
Consider the reaction below to answer the following question(s).

Refer to Exhibit 2-8.Label the acid and the base in the reaction.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
31
Exhibit 2-9
Indole is pleasant smelling in highly dilute solutions and has been used in perfumery.Use the structure of indole,below,to answer the following question(s).
Refer to Exhibit 2-9.Indole can function as a Lewis base in the presence of strong acid.Formulate a reaction,showing electron flow with arrows,that demonstrates this reactivity of indole.
Indole is pleasant smelling in highly dilute solutions and has been used in perfumery.Use the structure of indole,below,to answer the following question(s).

Refer to Exhibit 2-9.Indole can function as a Lewis base in the presence of strong acid.Formulate a reaction,showing electron flow with arrows,that demonstrates this reactivity of indole.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
32
Based on electronegativity values,in which of the following is the bond,represented by -,the most polar?
A)H3C-I
B)H3C-Na
C)H3C-Cl
D)H3C-OH
A)H3C-I
B)H3C-Na
C)H3C-Cl
D)H3C-OH

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
33
Draw two resonance structures for the species below. 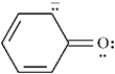


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
34
In which series are the elements listed in order of increasing electronegativity?
A)P < S < Cl
B)Ge < C < P
C)As < S < F
D)P < Br < N
A)P < S < Cl
B)Ge < C < P
C)As < S < F
D)P < Br < N

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
35
The condensed structure for dimethyl ether looks symmetrical.However,dimethyl ether has a dipole moment.Draw a structure that explains this and indicate the expected direction of the molecular dipole moment. 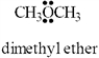
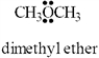

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
36
Use the curved arrow formalism to show the electron flow in the reaction of ammonia with water.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
37
Put a box around all the Lewis acids in the group of compounds below. 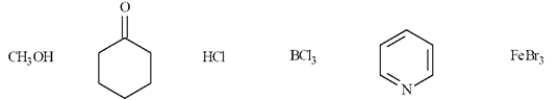
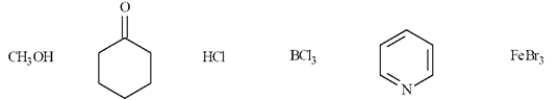

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
38
Exhibit 2-6
Refer to the following equation to answer the question(s) below.Place the letter corresponding to the correct answer in the blank.
Refer to Exhibit 2-6.The strongest Brønsted-Lowry base in the equation is ______.
Refer to the following equation to answer the question(s) below.Place the letter corresponding to the correct answer in the blank.

Refer to Exhibit 2-6.The strongest Brønsted-Lowry base in the equation is ______.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
39
Exhibit 2-10
Consider the acid-base reaction below to answer the following question(s).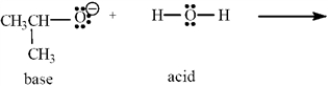
Refer to Exhibit 2-10.Write the products of this Lewis acid - base reaction.
Consider the acid-base reaction below to answer the following question(s).
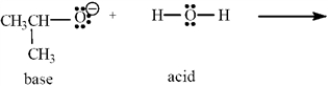
Refer to Exhibit 2-10.Write the products of this Lewis acid - base reaction.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
40
Exhibit 2-6
Refer to the following equation to answer the question(s) below.Place the letter corresponding to the correct answer in the blank.
Refer to Exhibit 2-6.The strongest Brønsted-Lowry acid in the equation is ______.
Refer to the following equation to answer the question(s) below.Place the letter corresponding to the correct answer in the blank.

Refer to Exhibit 2-6.The strongest Brønsted-Lowry acid in the equation is ______.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
41
How many resonance forms can be drawn for the NO3- ion?
A)1
B)2
C)3
D)4
E)None,the nitrate ion does not exhibit resonance.
A)1
B)2
C)3
D)4
E)None,the nitrate ion does not exhibit resonance.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
42
The following is generic depiction of a reaction using the curve arrow formalism. 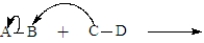 Which of these statements is not correct for this reaction?
Which of these statements is not correct for this reaction?
A)Electrons move from C to B.
B)Electrons move from B to A.
C)In the products,a bond forms between C and B.
D)In the products,A would have a positive charge.
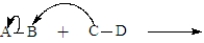 Which of these statements is not correct for this reaction?
Which of these statements is not correct for this reaction?A)Electrons move from C to B.
B)Electrons move from B to A.
C)In the products,a bond forms between C and B.
D)In the products,A would have a positive charge.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
43
Draw the resonance forms of 3,5-heptanedione anion.
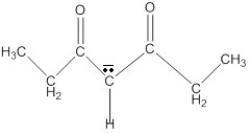


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
44
Draw the resonance forms of methyl acetate anion formed during the protonation of methyl acetate in the presence of sulfuric acid. 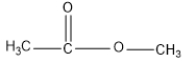
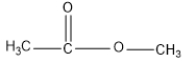

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
45
What are the products of the following reaction? 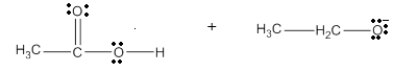
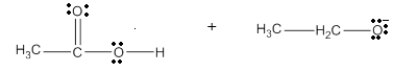

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
46
The structure for Vitamin K which is involved in blood clotting is shown below. 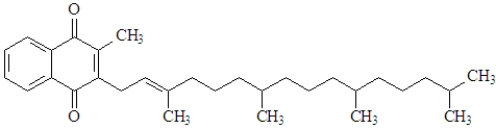 This vitamin would be:
This vitamin would be:
A)classified as hydrophilic.
B)water-soluble.
C)fat-soluble.
D)both hydrophilic and hydrophobic.
 This vitamin would be:
This vitamin would be:A)classified as hydrophilic.
B)water-soluble.
C)fat-soluble.
D)both hydrophilic and hydrophobic.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following substances has a zero dipole moment?
A)CO2
B)Cl2C=CCl2
C)HOCH2CH2OH
D)HCl2CCHCl2
E)All have zero dipole moments.
A)CO2
B)Cl2C=CCl2
C)HOCH2CH2OH
D)HCl2CCHCl2
E)All have zero dipole moments.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following would represent the strongest acid?
A)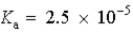
B)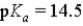
C)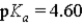
D)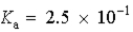
A)
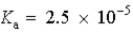
B)
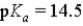
C)
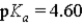
D)
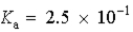

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following substances would be expected to have the largest pKa?
A)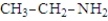
B)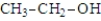
C)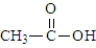
D)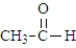
A)
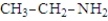
B)
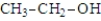
C)
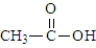
D)
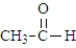

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following does not characterize the curved arrow formalism?
A)The arrow shows the movement of electrons not atoms.
B)The atom at the head of the arrow is the electron pair acceptor.
C)The atom at the tail of the arrow is a Lewis acid.
D)The species containing the atom at the head of the arrow will have the smaller pKa.
E)All of these correctly describe the curved arrow formalism.
A)The arrow shows the movement of electrons not atoms.
B)The atom at the head of the arrow is the electron pair acceptor.
C)The atom at the tail of the arrow is a Lewis acid.
D)The species containing the atom at the head of the arrow will have the smaller pKa.
E)All of these correctly describe the curved arrow formalism.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck


