
College Physics with MasteringPhysics 7th Edition by Jerry Wilson,Anthony Buffa,Bo Lou
Edition 7ISBN: 978-0321601834
College Physics with MasteringPhysics 7th Edition by Jerry Wilson,Anthony Buffa,Bo Lou
Edition 7ISBN: 978-0321601834 Exercise 9
An ideal gas initially at temperature T o , pressure p o , and volume V o is compressed to one-half its initial volume. As shown in Fig., process 1 is adiabatic, 2 is isothermal, and 3 is isobaric. Rank the work done on the gas and the final temperatures of the gas, from highest to lowest, for all three processes, and explain how you decided upon your rankings.
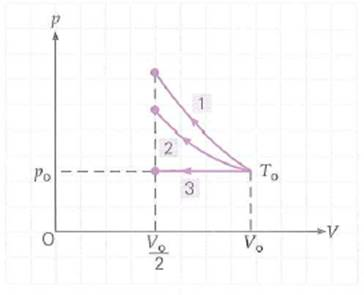
FIGURE Thermodynamic processes See Conceptual Question.

FIGURE Thermodynamic processes See Conceptual Question.
Explanation
Below plot pressure verses volume repres...
College Physics with MasteringPhysics 7th Edition by Jerry Wilson,Anthony Buffa,Bo Lou
Why don’t you like this exercise?
Other Minimum 8 character and maximum 255 character
Character 255


