
Campbell Biology 11th Edition by Lisa Urry,Michael Cain,Steven Wasserman,Peter Minorsky,Jane Reece
Edition 11ISBN: 978-0134093413
Campbell Biology 11th Edition by Lisa Urry,Michael Cain,Steven Wasserman,Peter Minorsky,Jane Reece
Edition 11ISBN: 978-0134093413 Exercise 10
Working with Moles and Molar Ratios
Could the First Biological Molecules Have Formed Near Volcanoes on Early Earth In 2007, Jeffrey Bada, a former graduate student of Stanley Miller, discovered some vials of samples that had never been analyzed from an experiment performed by Miller in 1958. In that experiment, Miller used hydrogen sulfide gas (H₂ S) as one of the gases in the reactant mixture. Since H₂ S is released by volcanoes, the H₂ S experiment was designed to mimic conditions near volcanoes on early Earth. In 2011, Bada and colleagues published the results of their analysis of these "lost" samples. In this exercise, you will make calculations using the molar ratios of reactants and products from the H₂ S experiment.
How the Experiment Was Done According to his laboratory notebook, Miller used the same apparatus as in his original experiment (see Figure 4.2), but the mixture of gaseous reactants included methane (CH₄), carbon dioxide (CO₂ ), hydrogen sulfide (H₂ S), and ammonia (NH 3 ). After three days of simulated volcanic activity, he collected samples of the liquid, partially purified the chemicals, and sealed the samples in sterile vials. In 2011, Bada's research team used modern analytical methods to analyze the products in the vials for the presence of amino acids, the building blocks of proteins.
Data from the Experiment The table below shows 4 of the 23 amino acids detected in the 2011 analysis of the samples from Miller's 1958 H₂ S experiment.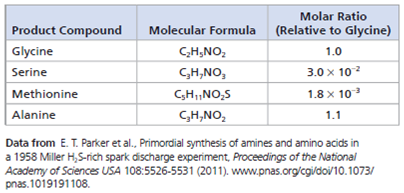

A mole is the number of particles of a substance with a mass equivalent to its molecular (or atomic) mass in daltons. There are 6.02 × 10 23 molecules (or atoms) in 1.0 mole (Avogadro's number; see Concept 3.2). The data table shows the "molar ratios" of some of the products from the Miller H₂ S experiment. In a molar ratio, each unitless value is expressed relative to a standard for that experiment. Here, the standard is the number of moles of the amino acid glycine, which is set to a value of 1.0. For instance, serine has a molar ratio of 3.0 × 10 2 , meaning that for every mole of glycine, there is 3.0 × 10 2 mole of serine.
(a) Give the molar ratio of methionine to glycine and explain what it means.
(b) How many molecules of glycine are present in 1.0 mole
(c) For every 1.0 mole of glycine in the sample, how many molecules of methionine are present (Recall that to multiply two numbers with exponents, you add their exponents; to divide them, you subtract the exponent in the denominator from that in the numerator.)
Could the First Biological Molecules Have Formed Near Volcanoes on Early Earth In 2007, Jeffrey Bada, a former graduate student of Stanley Miller, discovered some vials of samples that had never been analyzed from an experiment performed by Miller in 1958. In that experiment, Miller used hydrogen sulfide gas (H₂ S) as one of the gases in the reactant mixture. Since H₂ S is released by volcanoes, the H₂ S experiment was designed to mimic conditions near volcanoes on early Earth. In 2011, Bada and colleagues published the results of their analysis of these "lost" samples. In this exercise, you will make calculations using the molar ratios of reactants and products from the H₂ S experiment.
How the Experiment Was Done According to his laboratory notebook, Miller used the same apparatus as in his original experiment (see Figure 4.2), but the mixture of gaseous reactants included methane (CH₄), carbon dioxide (CO₂ ), hydrogen sulfide (H₂ S), and ammonia (NH 3 ). After three days of simulated volcanic activity, he collected samples of the liquid, partially purified the chemicals, and sealed the samples in sterile vials. In 2011, Bada's research team used modern analytical methods to analyze the products in the vials for the presence of amino acids, the building blocks of proteins.
Data from the Experiment The table below shows 4 of the 23 amino acids detected in the 2011 analysis of the samples from Miller's 1958 H₂ S experiment.


A mole is the number of particles of a substance with a mass equivalent to its molecular (or atomic) mass in daltons. There are 6.02 × 10 23 molecules (or atoms) in 1.0 mole (Avogadro's number; see Concept 3.2). The data table shows the "molar ratios" of some of the products from the Miller H₂ S experiment. In a molar ratio, each unitless value is expressed relative to a standard for that experiment. Here, the standard is the number of moles of the amino acid glycine, which is set to a value of 1.0. For instance, serine has a molar ratio of 3.0 × 10 2 , meaning that for every mole of glycine, there is 3.0 × 10 2 mole of serine.
(a) Give the molar ratio of methionine to glycine and explain what it means.
(b) How many molecules of glycine are present in 1.0 mole
(c) For every 1.0 mole of glycine in the sample, how many molecules of methionine are present (Recall that to multiply two numbers with exponents, you add their exponents; to divide them, you subtract the exponent in the denominator from that in the numerator.)
Explanation

This question doesn’t have an expert verified answer yet, let Examlex AI Copilot help.
Campbell Biology 11th Edition by Lisa Urry,Michael Cain,Steven Wasserman,Peter Minorsky,Jane Reece
Why don’t you like this exercise?
Other Minimum 8 character and maximum 255 character
Character 255


