
Molecular Biology Of The Cell 6th Edition by Bruce Alberts, Alexander Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Edition 6ISBN: 978-0815345244
Molecular Biology Of The Cell 6th Edition by Bruce Alberts, Alexander Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Edition 6ISBN: 978-0815345244 Exercise 1
Which statements are true? explain why or why not.
-The nuclear pore complex (NPC) creates a barrier to the free exchange of molecules between the nucleus and cytosol, but in a way that remains mysterious. In yeast, for example, the central pore of the NPC has a diameter of 35 nm and is 30 nm long, which is somewhat smaller than its vertebrate counterpart. Even so, it is large enough to accom- modate virtually all components of the cytosol. Yet the pore allows passive diffusion of molecules only up to about 40 kd; entry of anything larger requires help from a nuclear import receptor. Selective permeability is controlled by pro- tein components of the NPC that have unstructured, polar tails extending into the central pore. These tails are charac- terized by periodic repeats of the hydrophobic amino acids phenylalanine (F) and glycine (G). At high enough concentration (~50 mM), the FG-repeat domains of these proteins can form a gel, with a meshwork of interactions between the hydrophobic FG repeats (Figure Q12-2A). These gels allow passive diffu- sion of small molecules, but they prevent entry of larger proteins such as the fluorescent protein mCherry fused to maltose binding protein (MBP) (Figure Q12-2B). (The fusion to MBP makes mCherry too large to enter the nucleus by passive diffusion.) However, if the nuclear import receptor, importin, is fused to a similar protein, MBP-GFP, the importin-MBP-GFP fusion readily enters the gel (Figure Q12-2B).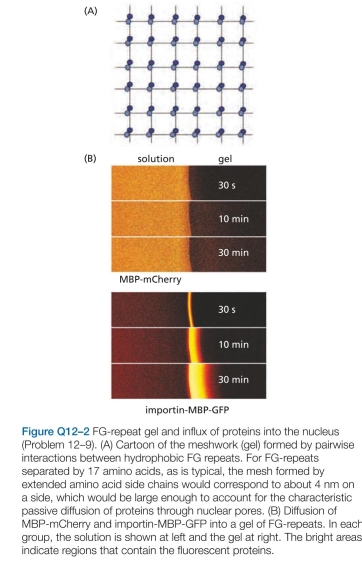 a. FG-repeats only form gels in vitro at relatively high concentration (50 mM). Is this concentration reasonable for FG repeats in the NPC core? In yeast, there are about 5000 FG-repeats in each NPC. Given the dimensions of the yeast nuclear pore (35 nm diameter and 30 nm length), calculate the concentration of FG-repeats in the cylindri- cal volume of the pore. Is this concentration comparable to the one used in vitro? b. A second question is whether the diffusion of importin-MBP-GFP through the FG-repeat gel is fast enough to account for the efficient flow of materials between the nucleus and cytosol. From experiments of the type shown in Figure Q12-2B, the diffusion coefficient (D) of importin-MBP-GFP through the FG-repeat gel was determined to be about
a. FG-repeats only form gels in vitro at relatively high concentration (50 mM). Is this concentration reasonable for FG repeats in the NPC core? In yeast, there are about 5000 FG-repeats in each NPC. Given the dimensions of the yeast nuclear pore (35 nm diameter and 30 nm length), calculate the concentration of FG-repeats in the cylindri- cal volume of the pore. Is this concentration comparable to the one used in vitro? b. A second question is whether the diffusion of importin-MBP-GFP through the FG-repeat gel is fast enough to account for the efficient flow of materials between the nucleus and cytosol. From experiments of the type shown in Figure Q12-2B, the diffusion coefficient (D) of importin-MBP-GFP through the FG-repeat gel was determined to be about 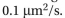 The equation for diffu- sion is
The equation for diffu- sion is 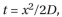 where t is time and x is distance. Calcu- late the time it would take importin-MBP-GFP to diffuse through a yeast nuclear pore (30 nm) if the pore consisted of a gel of FG-repeats. Does this time seem fast enough for the needs of a eukaryotic cell?
where t is time and x is distance. Calcu- late the time it would take importin-MBP-GFP to diffuse through a yeast nuclear pore (30 nm) if the pore consisted of a gel of FG-repeats. Does this time seem fast enough for the needs of a eukaryotic cell?
-The nuclear pore complex (NPC) creates a barrier to the free exchange of molecules between the nucleus and cytosol, but in a way that remains mysterious. In yeast, for example, the central pore of the NPC has a diameter of 35 nm and is 30 nm long, which is somewhat smaller than its vertebrate counterpart. Even so, it is large enough to accom- modate virtually all components of the cytosol. Yet the pore allows passive diffusion of molecules only up to about 40 kd; entry of anything larger requires help from a nuclear import receptor. Selective permeability is controlled by pro- tein components of the NPC that have unstructured, polar tails extending into the central pore. These tails are charac- terized by periodic repeats of the hydrophobic amino acids phenylalanine (F) and glycine (G). At high enough concentration (~50 mM), the FG-repeat domains of these proteins can form a gel, with a meshwork of interactions between the hydrophobic FG repeats (Figure Q12-2A). These gels allow passive diffu- sion of small molecules, but they prevent entry of larger proteins such as the fluorescent protein mCherry fused to maltose binding protein (MBP) (Figure Q12-2B). (The fusion to MBP makes mCherry too large to enter the nucleus by passive diffusion.) However, if the nuclear import receptor, importin, is fused to a similar protein, MBP-GFP, the importin-MBP-GFP fusion readily enters the gel (Figure Q12-2B).
 a. FG-repeats only form gels in vitro at relatively high concentration (50 mM). Is this concentration reasonable for FG repeats in the NPC core? In yeast, there are about 5000 FG-repeats in each NPC. Given the dimensions of the yeast nuclear pore (35 nm diameter and 30 nm length), calculate the concentration of FG-repeats in the cylindri- cal volume of the pore. Is this concentration comparable to the one used in vitro? b. A second question is whether the diffusion of importin-MBP-GFP through the FG-repeat gel is fast enough to account for the efficient flow of materials between the nucleus and cytosol. From experiments of the type shown in Figure Q12-2B, the diffusion coefficient (D) of importin-MBP-GFP through the FG-repeat gel was determined to be about
a. FG-repeats only form gels in vitro at relatively high concentration (50 mM). Is this concentration reasonable for FG repeats in the NPC core? In yeast, there are about 5000 FG-repeats in each NPC. Given the dimensions of the yeast nuclear pore (35 nm diameter and 30 nm length), calculate the concentration of FG-repeats in the cylindri- cal volume of the pore. Is this concentration comparable to the one used in vitro? b. A second question is whether the diffusion of importin-MBP-GFP through the FG-repeat gel is fast enough to account for the efficient flow of materials between the nucleus and cytosol. From experiments of the type shown in Figure Q12-2B, the diffusion coefficient (D) of importin-MBP-GFP through the FG-repeat gel was determined to be about 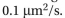 The equation for diffu- sion is
The equation for diffu- sion is 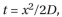 where t is time and x is distance. Calcu- late the time it would take importin-MBP-GFP to diffuse through a yeast nuclear pore (30 nm) if the pore consisted of a gel of FG-repeats. Does this time seem fast enough for the needs of a eukaryotic cell?
where t is time and x is distance. Calcu- late the time it would take importin-MBP-GFP to diffuse through a yeast nuclear pore (30 nm) if the pore consisted of a gel of FG-repeats. Does this time seem fast enough for the needs of a eukaryotic cell?Explanation
The statement, "In a bacterial genome, most of the Deoxyribonucleic acid (DNA) sequences code for proteins, but it is not the same in case of human genome." is true. Explanation: In a bacterial genome, most of the DNA sequences code for proteins. The remaining few sequences are involved in regulation of gene expression. They contain very less amount of noncoding DNA. Therefore, the most of the DNA sequences are involved in the mechanisms of reproduction, metabolism and other different functions. In case of human genome, proteins are coded by very small amount of DNA sequences. Even after allowing the sequences to regulate gene expression, most part of the human genome is composed of noncoding DNA. Hence, the statement, "In a bacterial genome, most of the Deoxyribonucleic acid (DNA) sequences code for proteins, but it is not the same in case of human genome." is true.
Molecular Biology Of The Cell 6th Edition by Bruce Alberts, Alexander Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Why don’t you like this exercise?
Other Minimum 8 character and maximum 255 character
Character 255


