Exam 2: Measurement
Exam 1: Foundations30 Questions
Exam 2: Measurement25 Questions
Exam 3: Atoms30 Questions
Exam 4: Light and Electronic Structure30 Questions
Exam 5: Chemical Bonds Compounds56 Questions
Exam 6: Chemical Reactions47 Questions
Exam 7: Mass Stoichiometry44 Questions
Exam 8: Energy39 Questions
Exam 9: Covalent Bonding and Molecules30 Questions
Exam 10: Solids, Liquids, and Gases30 Questions
Exam 11: Solutions30 Questions
Exam 12: Acids Bases30 Questions
Exam 13: Reaction Rates Equilibrium40 Questions
Exam 14: Oxidation-Reduction Reactions52 Questions
Exam 15: Organic Chemistry and Biomolecules65 Questions
Exam 16: Nuclear Chemistry52 Questions
Select questions type
A sample of metal has a mass of 0.0049 grams. What is this mass in milligrams?
Free
(Multiple Choice)
4.7/5  (38)
(38)
Correct Answer:
B
Normal average normal body temperature is 98.6 °F. Three children have their temperature taken at a doctor's office. The first child has a temperature of 310 K. The second child has a body temperature of 98.5 °F. The third child has a body temperature of 38.3 °C. Which child is running a fever (has a temperature greater than 100 °F)?
Free
(Multiple Choice)
4.8/5  (40)
(40)
Correct Answer:
C
A block of titanium metal has a mass of 1.22 kg. Given titanium's density (7.87 g/cm3), what volume does this block of titanium occupy in liters?
(Multiple Choice)
4.8/5  (38)
(38)
A piece of driftwood has a density of 0.76 g/cm3, while a piece of alloy has a density of 6.7 g/cm3. Which statement is accurate?
(Multiple Choice)
4.8/5  (41)
(41)
A student measures the volume of a solution to be 0.00370 L. How many significant digits are in this measurement?
(Multiple Choice)
4.9/5  (33)
(33)
A student measures the volume of a solution to be 0.03010 L. How many significant digits are in this measurement?
(Multiple Choice)
4.9/5  (36)
(36)
A sample of metal has a mass of 0.0793 kilograms. What is this mass in grams?
(Multiple Choice)
4.9/5  (43)
(43)
A candle made of a certain wax blend burns at a rate of 34.0 mg/min. What is the value of this burn rate if expressed in grams/hour?
(Multiple Choice)
4.9/5  (38)
(38)
The distribution of hits on the bull's-eye is described as: 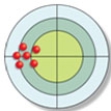
(Multiple Choice)
4.9/5  (44)
(44)
A 100.0-mL sample of lead has a much greater mass than a 100.0-mL sample of quartz. Select the accurate statement.
(Multiple Choice)
4.9/5  (31)
(31)
A solution has a mass of 17.41 grams and a volume of 14.4 mL. What is the density of this solution, reported to the correct number of significant digits?
(Multiple Choice)
4.9/5  (39)
(39)
The density of bromine is 3.12 g/mL. What is the mass of 155 mL of bromine?
(Multiple Choice)
4.8/5  (33)
(33)
A solution has a mass of 15.03 grams and a volume of 14.4 mL. What is the density of this solution, reported to the correct number of significant digits?
(Multiple Choice)
4.8/5  (43)
(43)
A car is moving at 60.0 mi/hr. How many feet/second is the driver traveling? (1 mile = 5,280 feet)
(Multiple Choice)
4.9/5  (44)
(44)
A block of titanium metal has a mass of 104.3 g. Given titanium's density (4.51 g/cm3), what volume does this block of titanium occupy in liters?
(Multiple Choice)
4.9/5  (35)
(35)
Showing 1 - 20 of 25
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)