Exam 3: Atomic Structure
Exam 1: Chemistry167 Questions
Exam 2: Atoms100 Questions
Exam 3: Atomic Structure190 Questions
Exam 4: Chemical Bonds189 Questions
Exam 5: Chemical Accounting103 Questions
Exam 6: Gases, Liquids, Solids, and Intermolecular Forces80 Questions
Exam 7: Acids and Bases126 Questions
Exam 8: Oxidation and Reduction116 Questions
Exam 9: Organic Chemistry186 Questions
Exam 10: Polymers136 Questions
Exam 11: Nuclear Chemistry122 Questions
Exam 12: Chemistry of the Earth106 Questions
Exam 13: Air146 Questions
Exam 14: Water117 Questions
Exam 15: Energy182 Questions
Exam 16: Biochemistry140 Questions
Exam 17: Food154 Questions
Exam 18: Drugs169 Questions
Exam 19: Fitness and Health104 Questions
Exam 20: Chemistry Down on the Farm70 Questions
Exam 21: Household Chemicals162 Questions
Exam 22: Poisons100 Questions
Select questions type
Solar fuels such as hydrogen are being studied as sources of energy. All of the following statements about hydrogen as a fuel source are true EXCEPT
(Multiple Choice)
4.9/5  (37)
(37)
Much of what we know about subatomic particles comes from directly observing the interior of the atom.
(True/False)
4.7/5  (39)
(39)
Which is a correct description of the organization of subatomic particles in atoms?
(Multiple Choice)
4.8/5  (24)
(24)
A carbon rod or metal strip inserted into a molten compound or a solution to carry the electric current is called an electrode.
(True/False)
4.7/5  (36)
(36)
When an electron in an excited atom "falls down" to a lower energy level, the excited atom emits a characteristic
(Multiple Choice)
5.0/5  (36)
(36)
The positive particles had varying masses in Goldstein's experiments using a gas discharge tube with perforated cathodes.
(True/False)
4.8/5  (38)
(38)
With the discovery of isotopes, which postulate of Dalton's original atomic theory must be modified?
(Multiple Choice)
4.8/5  (36)
(36)
Nuclear physicists have discovered over 100 different particles that compose the nucleus of an atom. From a chemistry perspective, the nucleus is best described as being composed of
(Multiple Choice)
4.8/5  (33)
(33)
Elements at the bottom of Group 7A on the periodic table are much more reactive than elements at the top of the group.
(True/False)
4.8/5  (48)
(48)
If X can represent the chemical symbol of any element in the periodic table, then 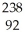 X represents an isotope of
X represents an isotope of
(Multiple Choice)
4.8/5  (40)
(40)
Showing 41 - 60 of 190
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)