Exam 20: Energy Transfer Processes and Thermodynamics
Exam 1: Physics and Measurement27 Questions
Exam 2: Motion in One Dimension48 Questions
Exam 3: Motion in Two Dimensions45 Questions
Exam 4: Forces and Newtons Laws47 Questions
Exam 5: Further Applications of Newtons Laws47 Questions
Exam 6: Work, Force and Energy46 Questions
Exam 7: Conservation of Energy46 Questions
Exam 8: Linear Momentum and Collisions47 Questions
Exam 9: Rotational Motion47 Questions
Exam 10: Energy and Momentum in Rotating Systems48 Questions
Exam 11: Gravity47 Questions
Exam 12: Special Relativity29 Questions
Exam 13: Fluid Statics38 Questions
Exam 14: Fluid Dynamics35 Questions
Exam 15: Solids37 Questions
Exam 16: Oscillatory Motion38 Questions
Exam 17: Wave Motion50 Questions
Exam 18: Superposition and Interference48 Questions
Exam 19: Heat and Temperature39 Questions
Exam 20: Energy Transfer Processes and Thermodynamics47 Questions
Exam 21: The Kinetic Theory of Gases35 Questions
Exam 22: The Second Law of Thermodynamics, Heat Engines and Entropy43 Questions
Select questions type
Two kilograms of water at 100 C is converted to steam at 1 atm. Find the change in internal energy (in J). (Lv = 2.26 *106 J/kg.)
Free
(Multiple Choice)
4.7/5  (35)
(35)
Correct Answer:
D
Determine the work done by 5 moles of an ideal gas that is kept at 100 C in an expansion from 1 litre to 5 litres.
Free
(Multiple Choice)
4.9/5  (42)
(42)
Correct Answer:
A
A team of people who travelled to the North Pole by dogsled lived on butter because they needed to consume 25 000 kilo-Joules each day. Because the ice there is lumpy and irregular, they had to help the dogs by pushing and lifting the load. Assume they had a 16-hour working day and that each person could lift a 500 N load. How many times would a person have to lift this weight 1.00 m upwards in a constant gravitational field where to do the work equivalent to 25 000 kJ?
Free
(Multiple Choice)
4.8/5  (31)
(31)
Correct Answer:
D
A 5-g coin is dropped from a 300-m building. If it reaches a terminal velocity of 45 m/s, and the rest of the energy is converted to heating the coin, what is the change in temperature (in C) of the coin? (The specific heat of copper is 387 J/kg. C.)
(Multiple Choice)
4.7/5  (34)
(34)
We are able to define a mechanical equivalent for heat because:
(Multiple Choice)
4.9/5  (34)
(34)
A child has a temperature of 101 F. If her total cross-sectional area is 2 m2, find the energy lost each second (in W) due to radiation, assuming the emissivity is 1. (Assume the room temperature is 70 F.)
(Multiple Choice)
4.9/5  (28)
(28)
A 100-kg student eats an 840-kJ doughnut. To 'burn it off', he decides to climb the steps of a tall building. How high (in m) would he have to climb to expend an equivalent amount of work?
(Multiple Choice)
4.7/5  (28)
(28)
The Earth intercepts 1.27 * 1017 W of radiant energy from the sun. Suppose the Earth, of volume 1.08 * 1021 m3, was composed of water. How long would it take for the Earth at 0 C to reach 100 C, if none of the energy was radiated or reflected back out into space?
(Multiple Choice)
4.7/5  (34)
(34)
A styrofoam container used as a Esky contains a block of ice at 0 C. If 225 grams of ice melts in 1 hour, how much heat energy per second is passing through the walls of the container? (The heat of fusion of ice is 3.33 * 105 J/kg).
(Short Answer)
4.9/5  (38)
(38)
Gas in a container increases its pressure from 1 atm to 3 atm while keeping its volume constant. Find the work done (in J) by the gas if the volume is 5 litres.
(Multiple Choice)
4.9/5  (36)
(36)
In a thermodynamic process, the internal energy of a system in a container with adiabatic walls decreases by 800 J. Which statement is correct?
(Multiple Choice)
4.9/5  (37)
(37)
Star A has a radius of 200 000 km and a surface temperature of 6000 K. Star B has a radius of 400 000 km and a surface temperature of 3000 K. The emissivity of both stars is the same. What is the ratio of the rate of energy radiated by Star A to that of Star B?
(Short Answer)
4.8/5  (25)
(25)
Five moles of an ideal gas expands isothermally at 100 C to five times its initial volume. Find the heat flow into the system.
(Multiple Choice)
4.8/5  (39)
(39)
A gas expands from A to B as shown in the graph. Calculate the work (in joules) done by the gas. (1 atm= 1.01 *105 N/m2.) 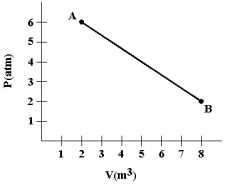
(Multiple Choice)
4.8/5  (25)
(25)
A cup of coffee is enclosed on all sides in an insulated container 1/2 cm thick in the shape of a cube 10 cm on a side. The temperature of the coffee is 95 C, and the temperature of the surroundings is 21 C. Find the rate of heat loss (in J/s) due to conduction if the thermal conductivity of the cup is 2 *10-4 cal/s.cm. C.
(Multiple Choice)
4.9/5  (38)
(38)
The R value of a particular brand of 'pink batt' roof insulation is 3 m2 K W-1. If the pink batts are 6cm thick, what is their thermal conductivity (in W m-1 K-1)?
(Multiple Choice)
4.8/5  (40)
(40)
Steel blocks A and B, which have equal masses, are at TA = 300 C and TB = 400 C. Block C, with mC = 2mA, is at TC = 350 C. Blocks A and B are placed in contact, isolated, and allowed to come into equilibrium. Then they are placed in contact with block C. At that instant:
(Multiple Choice)
4.9/5  (39)
(39)
The R-value of an insulating material is the thickness of the material divided by its thermal conductivity. When an insulating material consists of three layers with R-values R1, R2 and R3, the overall R-value of the insulation is given by:
(Multiple Choice)
4.8/5  (31)
(31)
The wall of a house consists of a slab of concrete (of R-value 0.5) with an insulating board (or R-value 1.5) firmly attached to the inside. The concrete and board are in thermal contact with each other. The temperature inside the house is 20 C and outside the house is 0 C. Determine the rate at which heat is lost through one square metre of wall (in W).
(Multiple Choice)
4.8/5  (37)
(37)
Showing 1 - 20 of 47
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)