Exam 3: Atomic Structure
Exam 1: Chemistry167 Questions
Exam 2: Atoms100 Questions
Exam 3: Atomic Structure190 Questions
Exam 4: Chemical Bonds189 Questions
Exam 5: Chemical Accounting103 Questions
Exam 6: Gases, Liquids, Solids, and Intermolecular Forces80 Questions
Exam 7: Acids and Bases126 Questions
Exam 8: Oxidation and Reduction116 Questions
Exam 9: Organic Chemistry186 Questions
Exam 10: Polymers136 Questions
Exam 11: Nuclear Chemistry122 Questions
Exam 12: Chemistry of the Earth106 Questions
Exam 13: Air146 Questions
Exam 14: Water117 Questions
Exam 15: Energy182 Questions
Exam 16: Biochemistry141 Questions
Exam 17: Food154 Questions
Exam 18: Drugs169 Questions
Exam 19: Fitness and Health104 Questions
Exam 20: Chemistry Down on the Farm70 Questions
Exam 21: Household Chemicals162 Questions
Exam 22: Poisons100 Questions
Select questions type
If X can represent the chemical symbol of any element in the periodic table,then 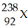 represents an isotope of
represents an isotope of
(Multiple Choice)
4.9/5  (34)
(34)
The mass of a nucleon is approximately four times the mass of a hydrogen atom.
(True/False)
4.8/5  (32)
(32)
The noble gases are a unique group of elements.They are recognized as unique due to the fact that they are
(Multiple Choice)
4.7/5  (33)
(33)
The nuclear model of the atom was constructed from which fundamental experiment?
(Multiple Choice)
4.8/5  (40)
(40)
With the discovery of isotopes,which postulate of Dalton's original atomic theory must be modified?
(Multiple Choice)
4.9/5  (37)
(37)
Which element has the same number of valence electrons as boron?
(Multiple Choice)
4.9/5  (43)
(43)
Which is a result of the mathematically based quantum view of electrons in atoms?
(Multiple Choice)
4.7/5  (37)
(37)
Which element is in the fourth period of the periodic table?
(Multiple Choice)
4.9/5  (38)
(38)
The electrons in the outermost energy level of an atom are called
(Multiple Choice)
4.8/5  (40)
(40)
Showing 61 - 80 of 190
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)