Exam 3: Solids, Fluids, Waves, Sound, Temperature, Thermal Expansion, and Ideal Gases
Exam 1: Measurements in Physics, Motion in One Dimension and Motion in Two Dimensions13 Questions
Exam 2: Force and Newtons Laws of Motion, Oscillations, Gravitation, Work and Energy16 Questions
Exam 3: Solids, Fluids, Waves, Sound, Temperature, Thermal Expansion, and Ideal Gases15 Questions
Exam 4: Heat, the Laws of Thermodynamics, Electric Charges, Forces, and Fields20 Questions
Exam 5: Electric Energy, Potential, Capacitors, Electric Current, Resistance, Circuits, Magnetic Fields and Forces14 Questions
Exam 6: Geometrical Optics, Electromagnetic Induction and Alternating Current15 Questions
Exam 7: Wave Optics and Modern Physics12 Questions
Exam 8: Nuclear Physics and Atomic Physics9 Questions
Exam 9: A Universe of Particles23 Questions
Select questions type
A standard atmosphere has a pressure versus altitude relation approximated by
? = Poe-?h,
With ? = 0.116 km-1. An aircraft flies at 30,000 ft but maintains a cabin pressure of that at
8000 ft. What force is exerted by air pressure on a square meter of cabin wall area?
Free
(Multiple Choice)
4.8/5  (33)
(33)
Correct Answer:
A
How many grams of ethanol (specific gravity 0.80) should be added to 5 grams of chloroform (specific gravity 1.50) if the resulting mixture is to have a specific gravity of 1.20?
Free
(Multiple Choice)
5.0/5  (44)
(44)
Correct Answer:
A
An ideal gas at STP is first compressed until its volume is half the initial volume, and then it is allowed to expand until its pressure is half the initial pressure. All of this is done while holding the temperature constant. If the initial internal energy of the gas is U, the final internal energy of the gas will be
Free
(Multiple Choice)
4.8/5  (41)
(41)
Correct Answer:
A
Transverse waves propagate at 43.2 m/s in a string that is subjected to a tension of 60.5 N. If the string is 15.9 m long, what is its mass?
(Multiple Choice)
4.9/5  (42)
(42)
What is the average kinetic energy of an ideal gas at 842 K? (The value of Boltzmann's constant is 1.38 × 10-23 J/K.)
(Multiple Choice)
4.8/5  (33)
(33)
How much would a lead brick 2.0 in × 2.0 in × 8.0 in weigh if placed in oil with density
? = 0.92 g/cm3? (?Pb = 11.4 g/cm3)
(Multiple Choice)
4.8/5  (33)
(33)
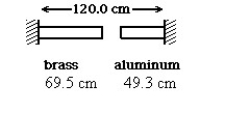 A brass rod is 69.5 cm long and an aluminum rod is 49.3 cm long when both rods are at an initial temperature of 0ºC. The rods are placed in line with a gap of 1.2 cm between them. The distance between the far ends of the rods is maintained at 120.0 cm throughout. The temperature is raised until the two rods are barely in contact. The coefficients of linear expansion of brass and aluminum are 2.0 × 10-5 K-1 and 2.4 × 10-5 K-1, respectively. In the figure, the temperature at which contact of the rods barely occurs, in °C, is closest to:
A brass rod is 69.5 cm long and an aluminum rod is 49.3 cm long when both rods are at an initial temperature of 0ºC. The rods are placed in line with a gap of 1.2 cm between them. The distance between the far ends of the rods is maintained at 120.0 cm throughout. The temperature is raised until the two rods are barely in contact. The coefficients of linear expansion of brass and aluminum are 2.0 × 10-5 K-1 and 2.4 × 10-5 K-1, respectively. In the figure, the temperature at which contact of the rods barely occurs, in °C, is closest to:
(Multiple Choice)
4.8/5  (34)
(34)
Find the displacement of a simple harmonic wave of amplitude 6.44 m at t = 0.71 s. Assume that the wave number is 2.34 m-1, the angular frequency is 2.88 rad/s, and that the wave is propagating in the +x direction at x = 1.21 m.
(Multiple Choice)
4.9/5  (29)
(29)
If you were to inhale a few breaths from a helium gas balloon, you would probably experience an amusing change in your voice. You would sound like Donald Duck or Alvin the Chipmunk. What is the cause of this curious high-pitched effect?
(Multiple Choice)
4.8/5  (37)
(37)
What is the total translational kinetic energy in a classroom filled with nitrogen at
1.01 × 105 Pa and 20.7ºC? The dimensions of the classroom are 4.60 m × 5.20 m × 8.80 m.
(Short Answer)
4.9/5  (46)
(46)
A 500.0 cm rope under a tension of 70.0 N is set into oscillation. The mass density of the rope is 120.0 g/cm. What is the frequency of the first harmonic mode (m = 2)?
(Multiple Choice)
4.9/5  (35)
(35)
Both the pressure and volume of a given sample of an ideal gas double. This means that its temperature in Kelvins must
(Multiple Choice)
4.9/5  (48)
(48)
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)