Exam 2: Basic Chemistry
Exam 1: The Human Body: An Orientation128 Questions
Exam 2: Basic Chemistry118 Questions
Exam 3: Cells and Tissues142 Questions
Exam 4: Skin and Body Membranes108 Questions
Exam 5: The Skeletal System124 Questions
Exam 6: The Muscular System129 Questions
Exam 7: The Nervous System161 Questions
Exam 8: Special Senses128 Questions
Exam 9: The Endocrine System149 Questions
Exam 10: Blood116 Questions
Exam 11: The Cardiovascular System160 Questions
Exam 12: The Lymphatic System and Body Defenses135 Questions
Exam 13: The Respiratory System150 Questions
Exam 14: The Digestive System and Body Metabolism162 Questions
Exam 15: The Urinary System138 Questions
Exam 16: The Reproductive System169 Questions
Select questions type
Which of these vitamins is produced in skin upon exposure to ultraviolet (UV) radiation?
(Multiple Choice)
4.9/5  (39)
(39)
The pH scale is based on the number of ________ in solution.
(Multiple Choice)
4.7/5  (30)
(30)
Match the following:
-Atomic number is based on the number of these subatomic particles in an atom of a particular element.
(Multiple Choice)
4.9/5  (42)
(42)
Discuss the organization of the pH scale, including the location of acids, bases, and neutral substances.
(Essay)
4.8/5  (35)
(35)
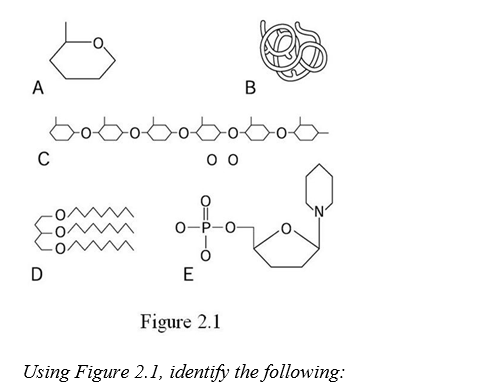 -Letter E represents a nucleic acid building block known as a ________.
-Letter E represents a nucleic acid building block known as a ________.
(Multiple Choice)
4.8/5  (40)
(40)
Shell 1 of an atom can hold a maximum of ________ electron(s).
(Multiple Choice)
4.9/5  (39)
(39)
Distinguish between a dehydration synthesis and a hydrolysis reaction.
(Essay)
4.7/5  (44)
(44)
Differentiate between the method of determination of the atomic number and the atomic mass number.
(Essay)
4.8/5  (32)
(32)
Joey works in a lab on an organic compound with the formula of C₆H₁₂O₆. Determine the type of organic compound, being as specific as possible, on which he works. Explain how you know.
(Essay)
4.8/5  (35)
(35)
The number of protons always equals the ________ in a neutral atom.
(Multiple Choice)
4.8/5  (39)
(39)
Which statement best describes why ATP is an important nucleic acid in the body?
(Multiple Choice)
4.8/5  (34)
(34)
The number of protons in an atom equals the atomic number for that element.
(True/False)
4.9/5  (41)
(41)
Describe the difference between the roles of functional, or globular, proteins and structural, or fibrous, proteins.
(Essay)
4.7/5  (41)
(41)
Hydrogen bonding between water molecules is responsible for ________.
(Multiple Choice)
4.7/5  (35)
(35)
Match the following:
-Amino acids join together to form proteins.
(Multiple Choice)
4.8/5  (41)
(41)
Unsaturated fatty acid chains contain one or more ________ bonds between carbon atoms.
(Multiple Choice)
4.7/5  (33)
(33)
Showing 21 - 40 of 118
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)