Exam 1: Matter, Measurement, and Problem Solving
Exam 1: Matter, Measurement, and Problem Solving120 Questions
Exam 2: Atoms and Elements116 Questions
Exam 3: Molecules, Compounds and Chemical Equations144 Questions
Exam 4: Chemical Quantities and Aqueous Reactions199 Questions
Exam 5: Gases157 Questions
Exam 6: Thermochemistry110 Questions
Exam 7: The Quantum-Mechanical Model of the Atom100 Questions
Exam 8: Periodic Properties of the Elements120 Questions
Exam 9: Chemical Bonding I: Lewis Theory125 Questions
Exam 10: Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and109 Questions
Exam 11: Liquids, Solids, and Intermolecular Forces123 Questions
Exam 12: Solutions127 Questions
Exam 13: Chemical Kinetics125 Questions
Exam 14: Chemical Equilibrium112 Questions
Exam 15: Acids and Bases126 Questions
Exam 16: Aqueous Ionic Equilibrium148 Questions
Exam 17: Free Energy and Thermodynamics103 Questions
Exam 18: Electrochemistry115 Questions
Exam 19: Radioactivity and Nuclear Chemistry105 Questions
Select questions type
Gas is sold for $1.399 per liter in Toronto,Canada.Your car needs 12.00 gallons.How much will your credit card be charged in dollars?
(Multiple Choice)
4.8/5  (41)
(41)
Two or more substances in variable proportions,where the composition is constant throughout are considered
(Multiple Choice)
4.7/5  (43)
(43)
If the temperature is 178°F,what is the temperature in degrees Celsius?
(Multiple Choice)
4.9/5  (34)
(34)
The density of air under ordinary conditions at 25°C is 1.19 g/L.How many kilograms of air are in a room that measures 11.0 ft × 11.0 ft and has a(n)10.0 ft ceiling? 1 in.= 2.54 cm (exactly);1 L = 103 cm3.
(Multiple Choice)
4.8/5  (45)
(45)
Because of the high heat and low humidity in the summer in Death Valley,California,a visitor requires about one quart of water for every two miles traveled on foot.Calculate the approximate number of liters required for a person to walk 10.kilometers in Death Valley.
(Multiple Choice)
4.8/5  (40)
(40)
Read the length of the metal bar with the correct number of significant figures. 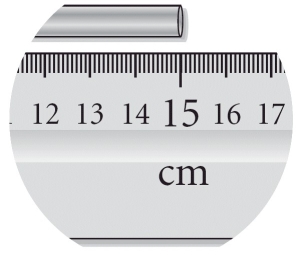
(Multiple Choice)
4.7/5  (40)
(40)
A student weighed 30.00 μg of sulfur in the lab.This is the same mass as
(Multiple Choice)
4.8/5  (34)
(34)
Read the length of the metal bar with the correct number of significant figures. 
(Multiple Choice)
4.8/5  (40)
(40)
A piece of metal ore weighs 8.25 g.When a student places it into a graduated cylinder containing water,the liquid level rises from 21.25 mL to 26.47 mL.What is the density of the ore?
(Multiple Choice)
4.8/5  (35)
(35)
What answer should be reported,with the correct number of significant figures,for the following calculation? (249.362 + 41)/ 63.498
(Multiple Choice)
4.7/5  (38)
(38)
Determine the volume of an object that has a mass of 455.6 g and a density of 19.3 g/cm3.
(Multiple Choice)
4.9/5  (42)
(42)
How many liters of air are in a room that measures 10.0 ft × 11.0 ft and has a(n)8.00 ft ceiling?
1 in.= 2.54 cm (exactly);1 L = 103 cm3.
(Multiple Choice)
4.9/5  (35)
(35)
How many of the following numbers contain 3 significant figures?
0.408 9.040 0.0400 9.05 × 1024
(Multiple Choice)
4.8/5  (34)
(34)
Showing 41 - 60 of 120
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)