Exam 2: Basic Chemistry of Cells
Exam 1: Biology, the Study of Life50 Questions
Exam 2: Basic Chemistry of Cells53 Questions
Exam 3: Organic Molecules of Cells54 Questions
Exam 4: Structure and Function of Cells56 Questions
Exam 5: Dynamic Activities of Cells50 Questions
Exam 6: Pathways of Photosynthesis51 Questions
Exam 7: Pathways of Cellular Respiration51 Questions
Exam 8: Cellular Reproduction50 Questions
Exam 9: Sexual Reproduction50 Questions
Exam 10: Patterns of Genetic Inheritance50 Questions
Exam 11: Molecular Biology of Inheritance49 Questions
Exam 12: Regulation of Gene Activity50 Questions
Exam 13: Biotechnology and Genomics52 Questions
Exam 14: Evidence of Evolution53 Questions
Exam 15: Speciation and Evolution49 Questions
Exam 16: The Evolutionary History of Life on Earth50 Questions
Exam 17: Evolution of Microbial Life50 Questions
Exam 18: Evolution of Protists50 Questions
Exam 19: Evolution of Plants and Fungi53 Questions
Exam 20: Evolution of Animals50 Questions
Exam 21: Evolution of Humans49 Questions
Exam 22: Plant Organization and Homeostasis50 Questions
Exam 23: Transport and Nutrition in Plants50 Questions
Exam 24: Control of Growth Responses in Plants50 Questions
Exam 25: Reproduction in Plants50 Questions
Exam 26: Animal Organization and Homeostasis63 Questions
Exam 27: Coordination by Neural Signaling64 Questions
Exam 28: Sense Organs64 Questions
Exam 29: Locomotion and Support Systems58 Questions
Exam 30: Circulation and Cardiovascular Systems59 Questions
Exam 31: Lymph Transport and Immunity57 Questions
Exam 32: Digestive Systems and Nutrition59 Questions
Exam 33: Gas Exchange and Transport in Animals56 Questions
Exam 34: Osmoregulation and Excretion57 Questions
Exam 35: Coordination by Hormone Signaling59 Questions
Exam 36: Reproduction and Development60 Questions
Exam 37: Population Ecology56 Questions
Exam 38: Behavioral Ecology59 Questions
Exam 39: Community and Ecosystem Ecology59 Questions
Exam 40: Major Ecosystems of the Biosphere58 Questions
Exam 41: Conservation Biology56 Questions
Select questions type
A neutral atom of phosphorus was found to have an atomic number of 15 and a mass number of 31.What is the total number of electrons in this atom?
Free
(Multiple Choice)
4.8/5  (28)
(28)
Correct Answer:
B
In order to determine if a patient has a thyroid tumor,which diagnostic procedure could be performed?
Free
(Multiple Choice)
4.9/5  (39)
(39)
Correct Answer:
D
When salt crystals dissolve in water,which chemical interactions in the salt crystals are being dissociated by the water molecules?
(Multiple Choice)
4.7/5  (42)
(42)
Which of the following elements is important in smaller quantities than is true for the six major elements in living things?
(Multiple Choice)
4.9/5  (43)
(43)
Which of the following is not a use of water by living organisms?
(Multiple Choice)
4.8/5  (30)
(30)
Which ions found specifically in bones and teeth are important in muscle contractions and nerve conduction?
(Multiple Choice)
4.8/5  (28)
(28)
Which term describes the tendency of water molecules to cling to other water molecules?
(Multiple Choice)
4.9/5  (30)
(30)
If a neutral oxygen atom gains two electrons from another atom,then the overall charge of this oxygen atom will become
(Multiple Choice)
4.9/5  (43)
(43)
Which factor determines whether an atom will be chemically reactive?
(Multiple Choice)
4.7/5  (29)
(29)
In a solution,if the hydroxide ion concentration exceeds the hydrogen ion concentration the solution is considered to be
(Multiple Choice)
4.9/5  (38)
(38)
Which kind of bond occurs between positively and negatively charged atoms?
(Multiple Choice)
4.8/5  (22)
(22)
The bond which results from a transfer of electrons from one atom to another is called an ionic bond.
(True/False)
4.8/5  (35)
(35)
Although high doses of radiation are harmful to cells,how can low levels of radioactive isotopes be used medicinally?
(Multiple Choice)
4.9/5  (37)
(37)
Two or more different elements bonded together is called an isotope.
(True/False)
4.7/5  (31)
(31)
Which six elements are the main components in living organisms?
(Multiple Choice)
4.8/5  (39)
(39)
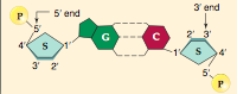 In a DNA molecule,the polar "G" nucleotide on one strand always bonds with the polar "C" nucleotide on the other strand.What kind of bonds are these?
In a DNA molecule,the polar "G" nucleotide on one strand always bonds with the polar "C" nucleotide on the other strand.What kind of bonds are these?
(Multiple Choice)
4.9/5  (44)
(44)
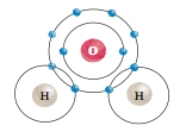 What type of chemical bond occurs specifically between one hydrogen atom and one oxygen atom in a water molecule?
What type of chemical bond occurs specifically between one hydrogen atom and one oxygen atom in a water molecule?
(Multiple Choice)
4.9/5  (39)
(39)
Showing 1 - 20 of 53
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)