Exam 2: Atoms and the Periodic Table
Exam 1: Matter and Measurements143 Questions
Exam 2: Atoms and the Periodic Table91 Questions
Exam 3: Ionic Compounds75 Questions
Exam 4: Molecular Compounds75 Questions
Exam 5: Classification and Balancing of Chemical Reactions53 Questions
Exam 6: Chemical Reactions: Mole and Mass Relationships46 Questions
Exam 7: Chemical Reactions: Energy, rates, and Equilibrium73 Questions
Exam 8: Gases, liquids, and Solids73 Questions
Exam 9: Solutions110 Questions
Exam 10: Acids and Bases102 Questions
Exam 11: Nuclear Chemistry88 Questions
Exam 12: Introduction to Organic Chemistry: Alkanes83 Questions
Exam 13: Alkenes, alkynes, and Aromatic Compounds85 Questions
Exam 14: Some Compounds With Oxygen, sulfur, or a Halogen94 Questions
Exam 15: Amines46 Questions
Exam 16: Aldehydes and Ketones77 Questions
Exam 17: Carboxylic Acids and Their Derivatives84 Questions
Exam 18: Amino Acids and Proteins78 Questions
Exam 19: Enzymes and Vitamins75 Questions
Exam 20: The Generation of Biochemical Energy66 Questions
Exam 21: Carbohydrates72 Questions
Exam 22: Carbohydrate Metabolism60 Questions
Exam 23: Lipids79 Questions
Exam 24: Lipid Metabolism58 Questions
Exam 25: Nucleic Acids and Protein Synthesis67 Questions
Exam 26: Genomics38 Questions
Exam 27: Protein and Amino Acid Metabolism47 Questions
Exam 28: Chemical Messengers: Hormones, neurotransmitters, and Drugs73 Questions
Exam 29: Body Fluids63 Questions
Select questions type
An atom containing 47 protons,47 electrons,and 60 neutrons has a mass number of
(Multiple Choice)
4.8/5  (35)
(35)
An atom that contains 47 protons,47 electrons,and 60 neutrons is an isotope of
(Multiple Choice)
4.8/5  (32)
(32)
Explain the term "quantized" as it applies to the energy of electrons.(Optional: Be sure to include an example that has not been previously mentioned of something that is quantized. )
(Essay)
4.8/5  (45)
(45)
In a neutral atom the number of ________ is equal to the number of ________.
(Multiple Choice)
4.8/5  (36)
(36)
The shell having n = 2 contains ________ subshells,________ orbitals,and up to ________ electrons.
(Multiple Choice)
4.8/5  (36)
(36)
The value for Z for an atom containing 47 protons,47 electrons,and 60 neutrons is
(Multiple Choice)
4.8/5  (37)
(37)
Protons possess a ________ charge,and neutrons possess a ________ charge.
(Multiple Choice)
4.9/5  (46)
(46)
Which particle has a mass approximately equal to the mass of a proton?
(Multiple Choice)
4.7/5  (36)
(36)
The element with the electron configuration 1s2 2s2 2p6 3s2 3p6 4s1 is
(Multiple Choice)
4.7/5  (31)
(31)
Cobalt is element 27.Cobalt-60 is used in the medical treatment of cancer.How many neutrons and protons are contained in the nucleus of this isotope?
(Multiple Choice)
4.7/5  (36)
(36)
Consider the isotope 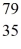 Br.The atomic number is ________,and the mass number is ________.
Br.The atomic number is ________,and the mass number is ________.
(Multiple Choice)
5.0/5  (35)
(35)
Valence electrons in the main group elements are contained in which type(s)of orbitals?
(Multiple Choice)
4.7/5  (36)
(36)
Showing 21 - 40 of 91
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)