Exam 2: Measurement and Problem Solving
Exam 1: The Chemical World54 Questions
Exam 2: Measurement and Problem Solving121 Questions
Exam 3: Matter and Energy110 Questions
Exam 4: Atoms and Elements106 Questions
Exam 5: Molecules and Compounds101 Questions
Exam 6: Chemical Composition111 Questions
Exam 7: Chemical Reactions102 Questions
Exam 8: Quantities in Chemical Reactions108 Questions
Exam 9: Electrons in Atoms and the Periodic Table101 Questions
Exam 10: Chemical Bonding100 Questions
Exam 11: Gases112 Questions
Exam 12: Liquids,solids,and Intermolecular Forces105 Questions
Exam 13: Solutions110 Questions
Exam 14: Acids and Bases106 Questions
Exam 15: Chemical Equilibrium106 Questions
Exam 16: Oxidation and Reduction102 Questions
Exam 17: Radioactivity and Nuclear Chemistry106 Questions
Exam 18: Organic Chemistry106 Questions
Exam 19: Biochemistry95 Questions
Select questions type
A conversion factor is a fraction with one unit on top and a different unit on the bottom.
(True/False)
4.8/5  (45)
(45)
A plastic block has dimensions of 2.2 cm × 3.0 cm × 1.5 cm and a mass of 12.4 grams.Will the block float in water and why?
(Multiple Choice)
4.8/5  (35)
(35)
When the number 2.35 is rounded to contain 2 significant figures it becomes 2.4.
(True/False)
4.8/5  (38)
(38)
The mass of an object,4.55 × 10-3 g,expressed in decimal notation is 0.000455 g.
(True/False)
4.9/5  (29)
(29)
Suppose a thermometer has marks at every one degree increment and the mercury level on the thermometer is exactly between the 25 and 26 degree Celsius marks.We should properly report the temperature measurement as
(Multiple Choice)
4.9/5  (43)
(43)
Given the following list of densities,which materials would float in a molten vat of lead provided that they do not themselves melt? Densities (g/mL): lead = 11.4,glass = 2.6,gold = 19.3,charcoal = 0.57,platinum = 21.4.
(Multiple Choice)
4.8/5  (35)
(35)
Determine the answer to the following equation with correct number of significant figures:
(17.103 + 2.03)× 1.02521 = ________
(Multiple Choice)
4.8/5  (42)
(42)
The correct number of significant figures in the number 0.002320 is:
(Multiple Choice)
4.7/5  (42)
(42)
How many significant digits should be reported in the answer to the following calculation? 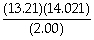 =
=
(Multiple Choice)
4.9/5  (38)
(38)
What is the volume of 12.8 g of a liquid that has a density of 0.789 g/mL?
(Multiple Choice)
5.0/5  (33)
(33)
Showing 101 - 120 of 121
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)