Exam 15: Radical Reactions
Exam 1: Structure and Bonding70 Questions
Exam 2: Acids and Bases48 Questions
Exam 3: Introduction to Organic Molecules and Functional Groups48 Questions
Exam 4: Alkanes56 Questions
Exam 5: Stereochemistry68 Questions
Exam 6: Understanding Organic Reactions43 Questions
Exam 7: Alkyl Halides and Nucleophilic Substitution64 Questions
Exam 8: Alkyl Halides and Elimination Reactions45 Questions
Exam 9: Alcohols, Ethers and Epoxides49 Questions
Exam 10: Alkenes47 Questions
Exam 11: Alkynes45 Questions
Exam 12: Oxidation and Reduction43 Questions
Exam 13: Mass Spectrometry and Infrared Spectroscopy42 Questions
Exam 14: Nuclear Magnetic Resonance Spectroscopy43 Questions
Exam 15: Radical Reactions43 Questions
Exam 16: Conjugation, Resonance, and Dienes44 Questions
Exam 17: Benzene and Aromatic Compounds40 Questions
Exam 18: Electrophilic Aromatic Substitution51 Questions
Exam 19: Carboxylic Acids and the Acidity of the O-H Bond44 Questions
Exam 20: Introduction to Carbonyl Chemistry; Organometallic Reagents; Oxidation and Reduction53 Questions
Exam 21: Aldehydes and Ketones Nucleophilic Addition45 Questions
Exam 22: Carboxylic Acids and Derivatives47 Questions
Exam 23: Substitution Reactions of Carbonyl Compounds at the Alpha-Carbon41 Questions
Exam 24: Carbonyl Condensation Reactions45 Questions
Exam 25: Amines58 Questions
Exam 26: Carbon-Carbon Bond Forming Reactions in Organic Synthesis41 Questions
Exam 27: Pericyclic Reactions52 Questions
Exam 28: Carbohydrates41 Questions
Exam 29: Amino Acids and Proteins41 Questions
Exam 30: Lipids41 Questions
Exam 31: Synthetic Polymers41 Questions
Select questions type
Which of the following alkenes undergoes allylic bromination to form a single monobrominated product? 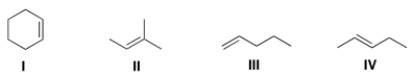
Free
(Multiple Choice)
5.0/5  (32)
(32)
Correct Answer:
A
What is the product of the following reaction? 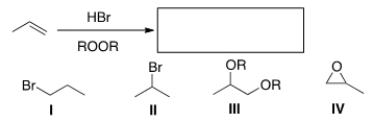
Free
(Multiple Choice)
4.8/5  (31)
(31)
Correct Answer:
A
How many monochlorination products can be formed (constitutional isomers only) from the reaction of CH3CH2CH2CH2CH2CH3 with Cl2 and h?
Free
(Multiple Choice)
4.8/5  (41)
(41)
Correct Answer:
A
Which of the indicated hydrogens is most readily abstracted in a free radical halogenation reaction? 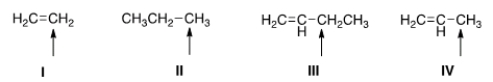
(Multiple Choice)
4.7/5  (40)
(40)
A possible reaction of ethane with chlorine is shown below. This reaction could conceivably occur by the following chain mechanisms [1], [2], and [3]. The chain initiating step(s) is (are) _____. [1] .
[2] .
[3] .
(Multiple Choice)
4.9/5  (43)
(43)
Which of the following statements about bromination is true?
(Multiple Choice)
4.8/5  (40)
(40)
How many monochlorination products can be formed from the reaction of (CH3)3CH with Cl2 and h?
(Multiple Choice)
4.7/5  (43)
(43)
Which of the following statements about radical reactions is not true?
(Multiple Choice)
4.8/5  (33)
(33)
What is the product in the following sequence of reactions? 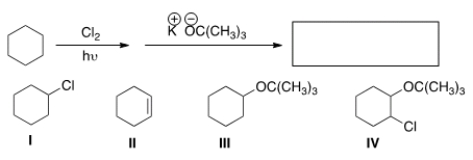
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following compounds contain secondary (2°) radical carbons? 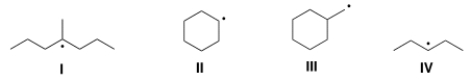
(Multiple Choice)
4.9/5  (26)
(26)
Which of the following statements about chlorination is true?
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following compounds contain tertiary (3°) radical carbons? 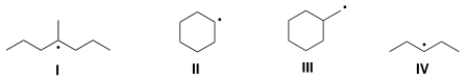
(Multiple Choice)
4.9/5  (42)
(42)
Rank the following radicals in order of increasing stability, putting the least stable first. 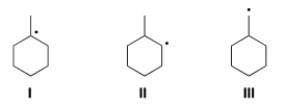
(Multiple Choice)
4.9/5  (40)
(40)
What type of reactive intermediate is formed in the reaction of propene with N-bromosuccinimide (NBS) to give 3-bromo-1-propene?
(Multiple Choice)
4.8/5  (41)
(41)
What is the product in the following sequence of reactions? 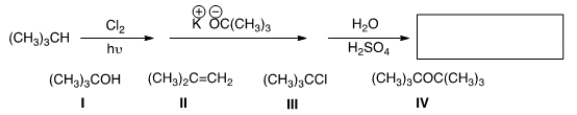
(Multiple Choice)
4.8/5  (30)
(30)
Select the route that would most likely produce the desired results from the given starting material.  I. (1) H2SO4 and heat; (2) HBr II. (1) KOH in ethanol; (2) HBr
III) (1) H2SO4 and heat; (2) HBr + peroxides
IV) (1) potassium tert-butoxide in tert-butanol; (2) HBr + peroxides
I. (1) H2SO4 and heat; (2) HBr II. (1) KOH in ethanol; (2) HBr
III) (1) H2SO4 and heat; (2) HBr + peroxides
IV) (1) potassium tert-butoxide in tert-butanol; (2) HBr + peroxides
(Multiple Choice)
4.8/5  (30)
(30)
How many monochlorination products (constitutional isomers and stereoisomers) are formed from the reaction of butane with Cl2 and h?
(Multiple Choice)
4.8/5  (41)
(41)
How many allylic halides can be formed when 3-methycyclohexene undergoes allylic halogenation with one equivalent of NBS and light?
(Multiple Choice)
4.8/5  (30)
(30)
Showing 1 - 20 of 43
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)