Exam 7: Chemical Quantities and Reactions
Exam 1: Chemistry in Our Lives52 Questions
Exam 2: Chemistry and Measurement85 Questions
Exam 3: Matter and Energy110 Questions
Exam 4: Atoms and Elements106 Questions
Exam 5: Nuclear Chemistry90 Questions
Exam 6: Ionic and Molecular Compounds106 Questions
Exam 7: Chemical Quantities and Reactions114 Questions
Exam 8: Gases76 Questions
Exam 9: Solutions73 Questions
Exam 10: Acids and Bases and Equilibrium67 Questions
Exam 11: Introduction to Organic Chemistry: Hydrocarbons123 Questions
Exam 12: Alcohols, thiols, ethers, aldehydes, and Ketones78 Questions
Exam 13: Carbohydrates81 Questions
Exam 14: Carboxylic Acids, esters, amines, and Amides103 Questions
Exam 15: Lipids65 Questions
Exam 16: Amino Acids, proteins, and Enzymes90 Questions
Exam 17: Nucleic Acids and Protein Synthesis64 Questions
Exam 18: Metabolic Pathways and Energy Production131 Questions
Select questions type
Barium chloride and sodium sulfate react according to the following equation.
BaCl2 + Na2SO4 → BaSO4 + 2NaCl
Answer the following question(s)about this reaction.
-How many grams of sodium chloride can be produced from 50.grams of barium chloride?
(Multiple Choice)
4.9/5  (33)
(33)
What coefficient is placed in front of O2 to complete the balancing of the following equation? C5H8 + ? O2 → 5CO2+ 4H2O
(Multiple Choice)
4.8/5  (34)
(34)
For the following question(s),consider the following equation.
2Mg + O2 → 2MgO
-How many grams of magnesium are needed to react with 16 g of O2 ?
(Multiple Choice)
4.9/5  (43)
(43)
If the reaction shown below is exothermic,the energy level of the reactants is ________.  +
+ 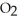 →
→  O
O
(Multiple Choice)
4.9/5  (37)
(37)
In this reaction,what is the correct coefficient for hydrogen gas? ? H2 + ? O2 → ? H2O
(Multiple Choice)
4.8/5  (41)
(41)
The ________ is the energy difference between reactants and products in a chemical reaction.
(Multiple Choice)
4.8/5  (33)
(33)
For the following question(s),consider the following balanced equation. Mg3N2+ 6H2O → 3Mg(OH)2 + 2NH3
-How many grams of  are produced when 125 g of
are produced when 125 g of  reacts as shown in the following balanced equation? 4
reacts as shown in the following balanced equation? 4  + 6NO → 5
+ 6NO → 5  + 6
+ 6  O
O
(Multiple Choice)
4.8/5  (32)
(32)
For the following question(s),consider the following balanced equation. Mg3N2+ 6H2O → 3Mg(OH)2 + 2NH3
-Find the mass of AlCl3 that is produced when 25.0 grams of Al2O3 reacts with HCl according to the following equation. Al2O3 + 6HCl → 2AlCl3 + 3H2O
(Multiple Choice)
4.9/5  (36)
(36)
When the following equation is balanced,the coefficient of O2 is ________.
CH4 + O2 → CO2 + H2O
(Short Answer)
4.8/5  (36)
(36)
For the following question(s),consider the following equation.
2Mg + O2 → 2MgO
-The number of moles of MgO produced when 0.20 mole of O2 reacts completely is ________.
(Multiple Choice)
4.8/5  (42)
(42)
What is the classification for this unbalanced reaction? Fe + HCl → FeCl3 + H2
(Multiple Choice)
4.8/5  (37)
(37)
In the following reaction,20.0 g of Al will react completely with 30.0 g of Br2.
2Al + 3  → 2
→ 2 
(True/False)
4.8/5  (36)
(36)
Showing 81 - 100 of 114
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)