Exam 2: Energy and Matter
Exam 1: Chemistry and Measurements77 Questions
Exam 2: Energy and Matter100 Questions
Exam 3: Atoms and Elements125 Questions
Exam 4: Nuclear Chemistry92 Questions
Exam 5: Compounds and Their Bonds115 Questions
Exam 6: Chemical Reactions and Quantities115 Questions
Exam 7: Gases100 Questions
Exam 8: Solutions99 Questions
Exam 9: Reaction Rates and Chemical Equilibrium71 Questions
Exam 10: Acids and Bases84 Questions
Exam 11: Introduction to Organic Chemistry: Alkanes117 Questions
Exam 12: Alkenes, Alkynes, and Aromatic Compounds81 Questions
Exam 13: Alcohols, Phenols, Thiols, and Ethers74 Questions
Exam 14: Aldehydes, Ketones, and Chiral Molecules83 Questions
Exam 15: Carbohydrates67 Questions
Exam 16: Carboxylic Acids and Esters85 Questions
Exam 17: Lipids83 Questions
Exam 18: Amines and Amides87 Questions
Exam 19: Amino Acids and Proteins103 Questions
Exam 20: Enzymes and Vitamins100 Questions
Exam 21: Nucleic Acids and Protein Synthesis89 Questions
Exam 22: Metabolic Pathways for Carbohydrates83 Questions
Exam 23: Metabolism and Energy Production86 Questions
Exam 24: Metabolic Pathways for Lipids and Amino Acids83 Questions
Select questions type
A burn from steam at 100 °C is expected to be more severe than a burn from boiling water at 100 °C because
(Multiple Choice)
4.8/5  (37)
(37)
Bromine (Br₂) has a freezing point of -7 °C, and a boiling point of 60 °C.
Indicate the state or change of state occurring at each temperature.
-30 °C
(Short Answer)
4.8/5  (39)
(39)
Bromine (Br₂) has a freezing point of -7 °C, and a boiling point of 60 °C.
Indicate the state or change of state occurring at each temperature.
--7 °C
(Short Answer)
5.0/5  (41)
(41)
Match the state of matter with each of the following descriptions of a substance.
-This material has a definite volume, and a definite shape.
(Multiple Choice)
4.7/5  (42)
(42)
A cheeseburger from a fast food restaurant contains 19 g of fat, 20 g of carbohydrate, and 28 g of protein. How many kcal of energy does the cheeseburger contain? (The accepted caloric values for foods are 4.0 kcal/g for carbohydrate, 9 kcal/g for fat, and 4 kcal/g for protein.) Report the answer to 2 significant figures.
(Multiple Choice)
4.9/5  (50)
(50)
On a hot day, the thermometer read 95 °F. What is the temperature in degrees Celsius?
(Multiple Choice)
4.9/5  (37)
(37)
Identify the physical state(s) corresponding to the regions on the cooling curve below. 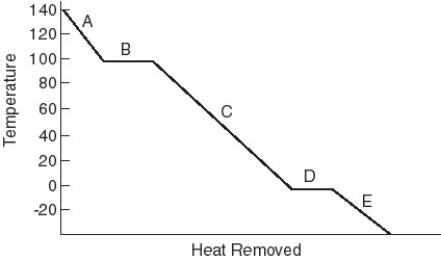 -B
A) solid and gas
B) liquid and gas
C) gas
D) liquid and solid
E) solid
F) liquid
-B
A) solid and gas
B) liquid and gas
C) gas
D) liquid and solid
E) solid
F) liquid
(Short Answer)
4.9/5  (45)
(45)
The specific heat of copper is 0.0920 cal/g °C, and the specific heat of silver is 0.0562 cal/g °C. If 100 cal of heat is added to one g of each metal at 25 °C, what is the expected result?
(Multiple Choice)
4.8/5  (31)
(31)
The energy stored in the chemical bonds of a carbohydrate molecule is
(Multiple Choice)
4.8/5  (32)
(32)
If the temperature is - 55 °C, what is the corresponding temperature on the Kelvin scale?
(Multiple Choice)
4.8/5  (32)
(32)
Identify each of the following transformations as a chemical or physical change
-baking a cake
(Multiple Choice)
5.0/5  (40)
(40)
The number of calories needed to raise the temperature of 32 g of water from 12 °C to 54 °C is
(Multiple Choice)
4.8/5  (40)
(40)
A kilocalorie of heat is required to raise the temperature of
(Multiple Choice)
4.9/5  (34)
(34)
Identify each of the following transformations as a chemical or physical change
-placing photographs in a scrapbook
(Multiple Choice)
4.8/5  (33)
(33)
Identify the physical state(s) corresponding to the regions on the cooling curve below.  -E
A) solid and gas
B) liquid and gas
C) gas
D) liquid and solid
E) solid
F) liquid
-E
A) solid and gas
B) liquid and gas
C) gas
D) liquid and solid
E) solid
F) liquid
(Short Answer)
4.8/5  (33)
(33)
Showing 41 - 60 of 100
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)