Exam 8: Acids and Bases
Exam 1: Matter, Energy, and Measurement143 Questions
Exam 2: Atoms133 Questions
Exam 3: Chemical Bonds140 Questions
Exam 4: Chemical Reactions140 Questions
Exam 5: Gases, Liquids, and Solids132 Questions
Exam 6: Solutions and Colloids153 Questions
Exam 7: Reaction Rates and Chemical Equilibrium103 Questions
Exam 8: Acids and Bases197 Questions
Exam 9: Nuclear Chemistry155 Questions
Exam 10: Organic Chemistry70 Questions
Exam 11: Alkanes142 Questions
Exam 12: Alkenes and Alkynes123 Questions
Exam 13: Benzene and Its Derivatives60 Questions
Exam 14: Alcohols, Ethers, and Thiols122 Questions
Exam 15: Chirality: the Handedness of Molecules92 Questions
Exam 16: Amines89 Questions
Exam 17: Aldehydes and Ketones101 Questions
Exam 18: Carboxylic Acids115 Questions
Exam 19: Carboxylic Anhydrides, Esters, and Amides117 Questions
Exam 20: Carbohydrates103 Questions
Exam 21: Lipids132 Questions
Exam 22: Proteins128 Questions
Exam 23: Enzymes62 Questions
Exam 24: Chemical Communications: Neurotransmitters and Hormones89 Questions
Exam 25: Nucleotides, Nucleic Acids, and Heredity121 Questions
Exam 26: Gene Expression and Protein Synthesis129 Questions
Exam 27: Bioenergetics: How the Body Converts Food to Energy140 Questions
Exam 28: Specific Catabolic Pathways: Carbohydrate, Lipid, and Protein Metabolism104 Questions
Exam 29: Biosynthetic Pathways67 Questions
Exam 30: Nutrition73 Questions
Exam 31: Immunochemistry142 Questions
Exam 32: Body Fluids72 Questions
Select questions type
Which of the following solutions will require the most NaOH in a titration experiment? Assume complete neutralization.
(Multiple Choice)
4.7/5  (35)
(35)
Examine the activity series given below.  -In the activity series shown, where are the strongest reducing agents located?
-In the activity series shown, where are the strongest reducing agents located?
(Multiple Choice)
4.9/5  (31)
(31)
What is the name of the acid which dissociates to give the cyanide ion, CN-?
(Multiple Choice)
4.9/5  (46)
(46)
An HCl solution has a pH of 3.10, what is the [OH-] in this solution?
(Multiple Choice)
4.7/5  (33)
(33)
The pKa of acetic acid, HC2H3O2, is 4.75. What is the pH of a buffer in which [HC2H3O2] = 0.20 M and [NaC2H3O2] = 2.0 M?
(Multiple Choice)
4.9/5  (32)
(32)
Which of the following is true about buffers such as HEPES, TRIS, MOPS, etc.?
(Multiple Choice)
4.9/5  (35)
(35)
When solid sodium hydroxide is dissolved in water which of the following species is not present in the solution?
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following is the conjugate base of acetic acid, CH3COOH?
(Multiple Choice)
4.8/5  (42)
(42)
Which of the following occurs when an acid reacts with an active metal?
(Multiple Choice)
4.9/5  (39)
(39)
When hydrogen chloride gas is dissolved in water which of the following species is not present in the solution?
(Multiple Choice)
4.8/5  (30)
(30)
Why does the label ammonium hydroxide on a bottle give a false impression about the bottle=s contents?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following solutions will require the most NaOH in a titration experiment?
(Multiple Choice)
4.9/5  (38)
(38)
The term "litmus test" is commonly used to mean a totally definitive test. In chemistry litmus is used as an acid-base indicator. Which of the following properly relates the chemical and common usages of the term "litmus test"?
(Multiple Choice)
4.9/5  (41)
(41)
When acetic acid is dissolved in water which of the following is true of the equilibrium which is established as represented below? 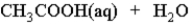

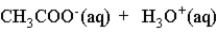
(Multiple Choice)
4.8/5  (41)
(41)
Examine the activity series given below.  -Examine the following image. Assume the solution in the beaker is a buffer solution.
-Examine the following image. Assume the solution in the beaker is a buffer solution.  What is the pOH of the solution in the beaker?
What is the pOH of the solution in the beaker?
(Multiple Choice)
4.8/5  (48)
(48)
Which of the following occurs when the weak base ammonia, NH3, is dissolved in water?
(Multiple Choice)
5.0/5  (40)
(40)
Which of the following gases is produced when an acid reacts with an active metal?
(Multiple Choice)
4.9/5  (42)
(42)
Which of the following is true of a buffer prepared with equal concentrations of an acid and its conjugate base?
(Multiple Choice)
5.0/5  (38)
(38)
A sodium hydroxide solution has a pH of 11.40, what is the [H3O+] in this solution?
(Multiple Choice)
4.8/5  (34)
(34)
Showing 21 - 40 of 197
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)