Deck 21: Heat Engines and Refrigerators
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/44
Play
Full screen (f)
Deck 21: Heat Engines and Refrigerators
1
A refrigerator removes heat from the freezing compartment at the rate of 20 kJ and ejects 24 kJ into a room per cycle.How much work is required in each cycle?
A)4 kJ
B)20 kJ
C)22 kJ
D)24 kJ
E)44 kJ
A)4 kJ
B)20 kJ
C)22 kJ
D)24 kJ
E)44 kJ
4 kJ
2
A real (non-Carnot)heat engine,operating between heat reservoirs at temperatures of 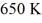 and
and 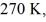 performs 4.3 kJ of net work and rejects
performs 4.3 kJ of net work and rejects 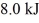 of heat in a single cycle.The thermal efficiency of this heat engine is closest to
of heat in a single cycle.The thermal efficiency of this heat engine is closest to
A)0.35.
B)0.31.
C)0.28.
D)0.38.
E)0.42.
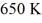 and
and 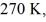 performs 4.3 kJ of net work and rejects
performs 4.3 kJ of net work and rejects 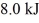 of heat in a single cycle.The thermal efficiency of this heat engine is closest to
of heat in a single cycle.The thermal efficiency of this heat engine is closest toA)0.35.
B)0.31.
C)0.28.
D)0.38.
E)0.42.
0.35.
3
A refrigerator has a coefficient of performance equal to 4.2.How much work must be done on the refrigerator in order to remove 250 J of heat from the interior?
A)60 J
B)120 J
C)250 J
D)480 J
E)1050 J
A)60 J
B)120 J
C)250 J
D)480 J
E)1050 J
60 J
4
Is it possible to transfer heat from a cold reservoir to a hot reservoir?
A)No; this is forbidden by the second law of thermodynamics.
B)Yes; this will happen naturally.
C)Yes,but work will have to be done.
D)Theoretically yes,but it hasn't been accomplished yet.
A)No; this is forbidden by the second law of thermodynamics.
B)Yes; this will happen naturally.
C)Yes,but work will have to be done.
D)Theoretically yes,but it hasn't been accomplished yet.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
5
During each cycle of operation,a refrigerator absorbs 230 J of heat from the freezer and expels 356 J of heat to the room.How much work input is required in each cycle?
A)712 J
B)586 J
C)460 J
D)310 J
E)126 J
A)712 J
B)586 J
C)460 J
D)310 J
E)126 J

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
6
An air conditioner with a coefficient of performance of 3.5 uses 30 kW of power.How much power is it discharging to the outdoors?
A)30 kW
B)75 kW
C)105 kW
D)135 kW
E)210 kW
A)30 kW
B)75 kW
C)105 kW
D)135 kW
E)210 kW

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
7
A heat engine takes 2.0 moles of an ideal gas through the reversible cycle abca,on the pV diagram shown in the figure.The path bc is an isothermal process.The temperature at c is 820 K,and the volumes at a and c are 0.010 m3 and 0.16 m3,respectively.The molar heat capacity at constant volume,of the gas,is 37 J/mol ∙ K,and the ideal gas constant is R = 8.314 J/(mol ∙ K).The thermal efficiency of the engine is closest to 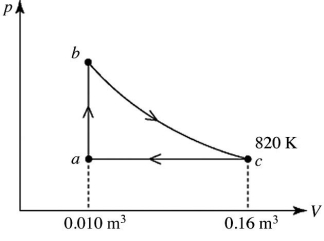
A)0.26.
B)0.026.
C)0.33.
D)0.40.
E)0.53.

A)0.26.
B)0.026.
C)0.33.
D)0.40.
E)0.53.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
8
An ideal reversible refrigerator keeps its inside compartment at 9.0°C.What is the high temperature,Th,needed to give this refrigerator a coefficient of performance of 3.7?
A)85°C
B)1052°C
C)11°C
D)42°C
A)85°C
B)1052°C
C)11°C
D)42°C

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
9
A Carnot engine operating between a reservoir of liquid mercury at its melting point (233 K)and a colder reservoir extracts 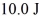 of heat from the mercury and does
of heat from the mercury and does 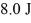 of work during each cycle.What is the temperature of the colder reservoir?
of work during each cycle.What is the temperature of the colder reservoir?
A)47 K
B)186 K
C)163 K
D)207 K
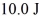 of heat from the mercury and does
of heat from the mercury and does 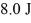 of work during each cycle.What is the temperature of the colder reservoir?
of work during each cycle.What is the temperature of the colder reservoir?A)47 K
B)186 K
C)163 K
D)207 K

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
10
A certain engine extracts 1300 J of heat from a hot temperature reservoir and discharges 700 J of heat to a cold temperature reservoir.What is the efficiency of this engine?
A)46%
B)54%
C)86%
D)27%
E)13%
A)46%
B)54%
C)86%
D)27%
E)13%

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
11
A heat engine with an efficiency of 30.0% performs 2500 J of work.How much heat is discharged to the lower temperature reservoir?
A)5830 J
B)8330 J
C)750 J
D)1350 J
E)7080 J
A)5830 J
B)8330 J
C)750 J
D)1350 J
E)7080 J

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
12
A certain Carnot heat pump transfers energy from the outside of a house,where the temperature is -15°C,to the inside of a room where the temperature is 21°C.If this heat pump runs off of electricity,what is the minimum rate at which it uses electrical energy to deliver
150 W to the inside room?
150 W to the inside room?

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
13
A refrigerator has a coefficient of performance of 1.15,and it extracts 7.95 J of heat from the cold reservoir during each cycle.
(a)How much work is done on the gas in each cycle?
(b)How much heat is exhausted into the hot reservoir in each cycle?
(a)How much work is done on the gas in each cycle?
(b)How much heat is exhausted into the hot reservoir in each cycle?

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
14
An automobile engine takes in 4000 J of heat and performs 1100 J of mechanical work in each cycle.
(a)Calculate the engine's efficiency.
(b)How much heat is "wasted" in each cycle?
(a)Calculate the engine's efficiency.
(b)How much heat is "wasted" in each cycle?

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
15
A Carnot engine operates between a high temperature reservoir at 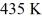 and a river with water at
and a river with water at 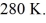 If it absorbs
If it absorbs 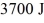 of heat each cycle,how much work per cycle does it perform?
of heat each cycle,how much work per cycle does it perform?
A)1318 J
B)2382 J
C)1449 J
D)2251 J
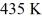 and a river with water at
and a river with water at 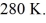 If it absorbs
If it absorbs 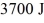 of heat each cycle,how much work per cycle does it perform?
of heat each cycle,how much work per cycle does it perform?A)1318 J
B)2382 J
C)1449 J
D)2251 J

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
16
The graph in the figure shows a cycle for a heat engine for which 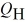 =35 J.What is the thermal efficiency of this engine?
=35 J.What is the thermal efficiency of this engine? 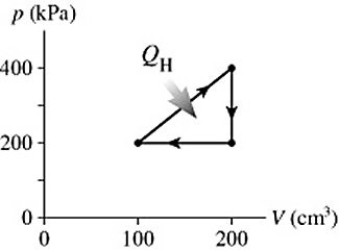
A)29 %
B)57 %
C)14 %
D)23 %
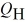 =35 J.What is the thermal efficiency of this engine?
=35 J.What is the thermal efficiency of this engine? 
A)29 %
B)57 %
C)14 %
D)23 %

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
17
A heat engine performs the reversible cycle abca with 9.0 moles of an ideal gas,as shown in the figure.Path ca is an adiabatic process.The temperatures at points a and b are 300 K and 500 K,respectively.The volume at point c is 0.20 m3.The adiabatic constant of the gas is 1.60.The thermal efficiency of this engine is closest to 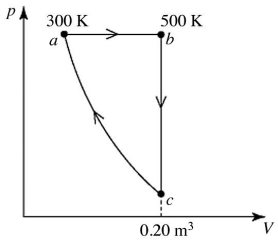
A)0.070.
B)0.10.
C)0.13.
D)0.16.
E)0.19.

A)0.070.
B)0.10.
C)0.13.
D)0.16.
E)0.19.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
18
Is it possible to transfer heat from a hot reservoir to a cold reservoir?
A)No; this is forbidden by the second law of thermodynamics.
B)Yes; this will happen naturally.
C)Yes,but work will have to be done.
D)Theoretically yes,but it hasn't been accomplished yet.
A)No; this is forbidden by the second law of thermodynamics.
B)Yes; this will happen naturally.
C)Yes,but work will have to be done.
D)Theoretically yes,but it hasn't been accomplished yet.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
19
An engine manufacturer makes the claim that the engine they have developed will,on each cycle,take 100 J of heat out of boiling water at 100°C,do mechanical work of 80 J,and exhaust 20 J of heat at 10°C.What,if anything,is wrong with this claim?
A)The heat exhausted must always be greater than the work done according to the second law of thermodynamics.
B)This engine violates the first law of thermodynamics because 100 J + 20 J ≠ 80 J.
C)An engine would operate by taking in heat at the lower temperature and exhausting heat at the higher temperature.
D)The efficiency of this engine is greater than the ideal Carnot cycle efficiency.
E)There is nothing wrong with this claim because 100 J = 20 J + 80 J.
A)The heat exhausted must always be greater than the work done according to the second law of thermodynamics.
B)This engine violates the first law of thermodynamics because 100 J + 20 J ≠ 80 J.
C)An engine would operate by taking in heat at the lower temperature and exhausting heat at the higher temperature.
D)The efficiency of this engine is greater than the ideal Carnot cycle efficiency.
E)There is nothing wrong with this claim because 100 J = 20 J + 80 J.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
20
A nuclear fission power plant has an actual efficiency of 39%.If 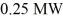 of power are produced by the nuclear fission,how much electric power does the power plant output?
of power are produced by the nuclear fission,how much electric power does the power plant output?
A)0.098 MW
B)9.8 MW
C)35 MW
D)0.35 MW
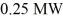 of power are produced by the nuclear fission,how much electric power does the power plant output?
of power are produced by the nuclear fission,how much electric power does the power plant output?A)0.098 MW
B)9.8 MW
C)35 MW
D)0.35 MW

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
21
A Carnot engine is operated as a heat pump to heat a room in the winter.The heat pump delivers heat to the room at the rate of 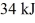 per second and maintains the room at a temperature of
per second and maintains the room at a temperature of 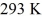 when the outside temperature is
when the outside temperature is 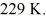 The power requirement for the heat pump under these operating conditions is closest to
The power requirement for the heat pump under these operating conditions is closest to
A)7500 W.
B)6000 W.
C)17,000 W.
D)13,000 W.
E)9600 W.
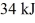 per second and maintains the room at a temperature of
per second and maintains the room at a temperature of 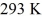 when the outside temperature is
when the outside temperature is 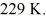 The power requirement for the heat pump under these operating conditions is closest to
The power requirement for the heat pump under these operating conditions is closest toA)7500 W.
B)6000 W.
C)17,000 W.
D)13,000 W.
E)9600 W.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
22
A Carnot cycle engine operates between a low temperature reservoir at 20°C and a high temperature reservoir at 800°C.If the engine is required to output 20.0 kJ of work per cycle,how much heat must the high temperature reservoir transfer to the engine during each cycle?
A)27.5 kJ
B)73.2 kJ
C)39.2 kJ
D)800 kJ
E)20.5 kJ
A)27.5 kJ
B)73.2 kJ
C)39.2 kJ
D)800 kJ
E)20.5 kJ

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
23
A system consists of two very large thermal reservoirs in contact with each other,one at temperature 300°C and the other at temperature 200°C.When 600 J of heat transfers from the 300°C reservoir to the 200°C reservoir,what is the change in entropy of this system?
A)0.221 J/K
B)1.00 J/K
C)5.00 J/K
D)-1.00 J/K
E)-2.31 J/K
A)0.221 J/K
B)1.00 J/K
C)5.00 J/K
D)-1.00 J/K
E)-2.31 J/K

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
24
A 610-g quantity of an ideal gas undergoes a reversible isothermal compression at a temperature of 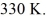 The compression reduces the volume of the gas from
The compression reduces the volume of the gas from 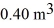 initially,to
initially,to 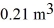 finally.The molecular mass of the gas is
finally.The molecular mass of the gas is 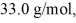 and the ideal gas constant is R = 8.314 J/(mol ∙ K).The entropy change for the gas is closest to
and the ideal gas constant is R = 8.314 J/(mol ∙ K).The entropy change for the gas is closest to
A)-99 J/K.
B)-81 J/K.
C)99 J/K.
D)81 J/K.
E)0.00 J/K.
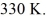 The compression reduces the volume of the gas from
The compression reduces the volume of the gas from 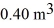 initially,to
initially,to 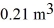 finally.The molecular mass of the gas is
finally.The molecular mass of the gas is 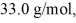 and the ideal gas constant is R = 8.314 J/(mol ∙ K).The entropy change for the gas is closest to
and the ideal gas constant is R = 8.314 J/(mol ∙ K).The entropy change for the gas is closest toA)-99 J/K.
B)-81 J/K.
C)99 J/K.
D)81 J/K.
E)0.00 J/K.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
25
What is the change in entropy of 10.0 moles of ideal monatomic gas that reversibly undergoes the isothermal expansion shown in the figure? The ideal gas constant is R = 8.314 J/(mol ∙ K). 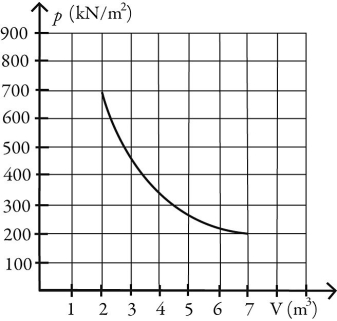
A)221 J/K
B)104 J/K
C)63.1 J/K
D)45.2 J/K
E)90.8 J/K

A)221 J/K
B)104 J/K
C)63.1 J/K
D)45.2 J/K
E)90.8 J/K

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
26
A 2.00 kg piece of lead at 40.0°C is placed in a very large quantity of water at 10.0°C,and thermal equilibrium is eventually reached.Calculate the entropy change of the lead that occurs during this process.The specific heat of lead is 130 J/(kg ∙ K).
A)-12.5 J/K
B)86.0 J/K
C)-26.2 J/K
D)-86.0 J/K
E)-6.24 J/K
A)-12.5 J/K
B)86.0 J/K
C)-26.2 J/K
D)-86.0 J/K
E)-6.24 J/K

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
27
A Carnot air conditioner operates between an indoor temperature of 20°C and an outdoor temperature of 39°C.How much energy does it need to remove 2000 J of heat from the interior of the house?
A)105 J
B)130 J
C)780 J
D)520 J
E)340 J
A)105 J
B)130 J
C)780 J
D)520 J
E)340 J

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
28
The compressor in a certain Carnot refrigerator performs 480 J of work to remove 150 J of heat from the interior of the refrigerator.How much heat must the coils behind the refrigerator discharge into the kitchen?
A)110 J
B)150 J
C)330 J
D)480 J
E)630 J
A)110 J
B)150 J
C)330 J
D)480 J
E)630 J

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
29
What is the maximum theoretical efficiency possible for a heat engine operating between a reservoir in which ice and water coexist,and a reservoir in which water and steam coexist? The pressure is constant at 1.0 atmosphere for both reservoirs.
A)27%
B)45%
C)73%
D)17%
A)27%
B)45%
C)73%
D)17%

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
30
An ideal Carnot engine operates between reservoirs having temperatures of 125°C and
-20°C.Each cycle the heat expelled by this engine is used to melt 30.0 g of ice at 0.00°C.The heat of fusion of water is 3.34 × 105 J/kg and the heat of vaporization of water is
2.25 × 106 J/kg.
(a)How much work does this engine do each cycle?
(b)How much heat per cycle does this engine absorb at the hot reservoir?
-20°C.Each cycle the heat expelled by this engine is used to melt 30.0 g of ice at 0.00°C.The heat of fusion of water is 3.34 × 105 J/kg and the heat of vaporization of water is
2.25 × 106 J/kg.
(a)How much work does this engine do each cycle?
(b)How much heat per cycle does this engine absorb at the hot reservoir?

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
31
One of the most efficient engines built so far has the following characteristics:
combustion chamber temperature = 1900°C
exhaust temperature = 430°C
7.0 × 109 cal of fuel produces 1.4 × 1010 J of work in one hour,where 1 cal = 4.19 J
(a)What is the actual efficiency of this engine?
(b)What is the Carnot efficiency of the engine?
(c)What is the power output of this engine?
combustion chamber temperature = 1900°C
exhaust temperature = 430°C
7.0 × 109 cal of fuel produces 1.4 × 1010 J of work in one hour,where 1 cal = 4.19 J
(a)What is the actual efficiency of this engine?
(b)What is the Carnot efficiency of the engine?
(c)What is the power output of this engine?

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
32
A coal-fired plant generates 600 MW of electric power.The plant uses 4.8 × 106 kg of coal each day.The heat produced by the combustion of coal is 3.3 × 107 J/kg.The steam that drives the turbines is at a temperature of 300°C,and the exhaust water is at 37°C.
(a)What is the overall efficiency of the plant for generating electric power?
(b)What is the maximum efficiency that this plant could possibly have using the same temperature extremes that it presently uses?
(c)How much thermal energy is exhausted each day by this plant?
(a)What is the overall efficiency of the plant for generating electric power?
(b)What is the maximum efficiency that this plant could possibly have using the same temperature extremes that it presently uses?
(c)How much thermal energy is exhausted each day by this plant?

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
33
You want to design an ideal Carnot heat engine that wastes only 35.0% of the heat that goes into it.The lowest cold-reservoir temperature available to you is +15.0°C.If 150.0 J of work is done per cycle,the heat input per cycle is closest to
A)203 J.
B)231 J.
C)248 J.
D)429 J.
E)760 J.
A)203 J.
B)231 J.
C)248 J.
D)429 J.
E)760 J.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
34
A perfect Carnot engine operates between the temperatures of 300K and 700K,drawing 60 kJ of heat from the 700K reservoir in each cycle.How much heat is dumped into the 300K reservoir in each cycle?
A)38 kJ
B)34 kJ
C)30 kJ
D)26 kJ
E)42 kJ
A)38 kJ
B)34 kJ
C)30 kJ
D)26 kJ
E)42 kJ

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
35
A 2.00 kg piece of lead at 40.0°C is placed in a very large quantity of water at 10.0°C,and thermal equilibrium is eventually reached.Calculate the TOTAL change in entropy that occurs during this process.The specific heat of lead is 130 J/(kg ∙ K).
A)190 J/K
B)100 J/K
C)6.6 J/K
D)6.2 J/K
E)1.4 J/K
A)190 J/K
B)100 J/K
C)6.6 J/K
D)6.2 J/K
E)1.4 J/K

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
36
The temperature inside a Carnot refrigerator placed in a kitchen at 22.0°C is 2.0°C.The heat extracted from the refrigerator is 89 MJ/h.What power is needed to operate this refrigerator?
A)1.7 kW
B)1.8 kW
C)1.5 kW
D)1.9 kW
E)1.6 kW
A)1.7 kW
B)1.8 kW
C)1.5 kW
D)1.9 kW
E)1.6 kW

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
37
A Carnot engine operates between reservoirs at 550K and 300K,discarding 1500 J of heat in each cycle.
(a)What is the engine's efficiency?
(b)How much heat is supplied to the engine by the hot reservoir in each cycle?
(a)What is the engine's efficiency?
(b)How much heat is supplied to the engine by the hot reservoir in each cycle?

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
38
A Carnot refrigerator has a coefficient of performance of 2.5.The refrigerator consumes 50 W of power.How much heat is removed from the interior of the refrigerator in 1 hour?
A)7.5 kJ
B)450 kJ
C)180 kJ
D)720 kJ
E)72 kJ
A)7.5 kJ
B)450 kJ
C)180 kJ
D)720 kJ
E)72 kJ

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
39
A Carnot refrigerator takes heat from water at 0°C and rejects heat to a room at 12°C.Suppose that 92.0 grams of water at 0°C are converted to ice at 0°C by the refrigerator.Calculate the mechanical energy that must be supplied to the refrigerator.The heat of fusion of water is 3.34 × 105 J/kg.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
40
A 2.0-kg block of aluminum at 50°C is dropped into 5.0 kg of water at 20°C and the temperature is allowed to stabilize.What is the total change in entropy during this process,assuming no heat is exchanged with the environment? The specific heat of aluminum is 910 J/(kg ∙ K)and the specific heat of water is 4190 J/(kg ∙ K).
A)8.2 J/K
B)10 J/K
C)3.3 × 10-2 J/K
D)3.8 × 10-3 J/K
E)2.4 × 10-3 J/K
A)8.2 J/K
B)10 J/K
C)3.3 × 10-2 J/K
D)3.8 × 10-3 J/K
E)2.4 × 10-3 J/K

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
41
A 810-g quantity of ethanol,in the liquid state at its melting point of 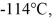 is frozen at atmospheric pressure.The heat of fusion of ethanol is 1.04 × 105 J/kg,the molecular mass is 46.1 g/mol,and the ideal gas constant is R = 8.314 J/(mol ∙ K).The change in the entropy of the ethanol as it freezes is closest to
is frozen at atmospheric pressure.The heat of fusion of ethanol is 1.04 × 105 J/kg,the molecular mass is 46.1 g/mol,and the ideal gas constant is R = 8.314 J/(mol ∙ K).The change in the entropy of the ethanol as it freezes is closest to
A)-540 J/K.
B)-490 J/K.
C)-600 J/K.
D)490 J/K.
E)540 J/K.
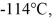 is frozen at atmospheric pressure.The heat of fusion of ethanol is 1.04 × 105 J/kg,the molecular mass is 46.1 g/mol,and the ideal gas constant is R = 8.314 J/(mol ∙ K).The change in the entropy of the ethanol as it freezes is closest to
is frozen at atmospheric pressure.The heat of fusion of ethanol is 1.04 × 105 J/kg,the molecular mass is 46.1 g/mol,and the ideal gas constant is R = 8.314 J/(mol ∙ K).The change in the entropy of the ethanol as it freezes is closest toA)-540 J/K.
B)-490 J/K.
C)-600 J/K.
D)490 J/K.
E)540 J/K.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
42
A brass rod,75.0 cm long and having a cross-sectional area of 2.50 cm2,conducts heat from a large furnace at 375°C into a very large cold water bath at 10.0°C without losing any heat at the lateral surface of the rod.Steady state has been established,and the thermal conductivity of brass is 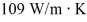 .The rate at which the entropy of the system (furnace plus water)is changing is closest to
.The rate at which the entropy of the system (furnace plus water)is changing is closest to
A)2.05 × 10-2 W/K.
B)2.64 × 10-2 W/K.
C)3.54 × 10-2 W/K.
D)4.69 × 10-2 W/K.
E)6.74 × 10-2 W/K.
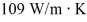 .The rate at which the entropy of the system (furnace plus water)is changing is closest to
.The rate at which the entropy of the system (furnace plus water)is changing is closest toA)2.05 × 10-2 W/K.
B)2.64 × 10-2 W/K.
C)3.54 × 10-2 W/K.
D)4.69 × 10-2 W/K.
E)6.74 × 10-2 W/K.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
43
A 2.00-kg block of ice at 0.00°C is dropped into a very large lake at 25.0°C and completely melts.For water,the heat of fusion is 3.35 × 105 J/kg,the heat of vaporization is 2.25 × 105 J/kg,and the specific heat is 4190 J/kg ∙ K.The net change in entropy of the system consisting of the ice and the lake due to this melting process is closest to
A)2.45 × 103 J/K.
B)2.24 × 103 J/K.
C)2.06 × 102 J/K.
D)-2.45 × 103 J/K.
E)-2.06 × 102 J/K.
A)2.45 × 103 J/K.
B)2.24 × 103 J/K.
C)2.06 × 102 J/K.
D)-2.45 × 103 J/K.
E)-2.06 × 102 J/K.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
44
At atmospheric pressure,45 moles of liquid helium are vaporized at its boiling point of 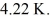 The heat of vaporization of helium,at atmospheric pressure,is
The heat of vaporization of helium,at atmospheric pressure,is 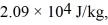 and the atomic weight of helium is
and the atomic weight of helium is 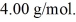 The change in the entropy of the helium,as it vaporizes,is closest to
The change in the entropy of the helium,as it vaporizes,is closest to
A)890 J/K.
B)14,000 J/K.
C)18,000 J/K.
D)-9400 J/K.
E)-14,000 J/K.
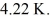 The heat of vaporization of helium,at atmospheric pressure,is
The heat of vaporization of helium,at atmospheric pressure,is 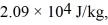 and the atomic weight of helium is
and the atomic weight of helium is 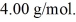 The change in the entropy of the helium,as it vaporizes,is closest to
The change in the entropy of the helium,as it vaporizes,is closest toA)890 J/K.
B)14,000 J/K.
C)18,000 J/K.
D)-9400 J/K.
E)-14,000 J/K.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck


