Exam 21: Heat Engines and Refrigerators
Exam 1: Concepts of Motion52 Questions
Exam 2: Kinematics in One Dimension59 Questions
Exam 3: Vectors and Coordinate Systems33 Questions
Exam 4: Kinematics in Two Dimensions49 Questions
Exam 5: Force and Motion30 Questions
Exam 6: Dynamics I: Motion Along a Line46 Questions
Exam 7: Newtons Third Law43 Questions
Exam 8: Dynamics II: Motion in a Plane20 Questions
Exam 9: Work and Kinetic Energy66 Questions
Exam 10: Interactions and Potential Energy55 Questions
Exam 11: Impulse and Momentum43 Questions
Exam 12: Rotation of a Rigid Body116 Questions
Exam 13: Newtons Theory of Gravity50 Questions
Exam 14: Fluids and Elasticity72 Questions
Exam 15: Oscillations49 Questions
Exam 16: Traveling Waves51 Questions
Exam 17: Superposition51 Questions
Exam 18: A Macroscopic Description of Matter46 Questions
Exam 19: Work, heat, and the First Law of Thermodynamics96 Questions
Exam 20: The Micromacro Connection41 Questions
Exam 21: Heat Engines and Refrigerators44 Questions
Exam 22: Electric Charges and Forces26 Questions
Exam 23: The Electric Field32 Questions
Exam 24: Gausss Law41 Questions
Exam 25: The Electric Potential40 Questions
Exam 26: Potential and Field57 Questions
Exam 27: Current and Resistance32 Questions
Exam 28: Fundamentals of Circuits68 Questions
Exam 29: The Magnetic Field83 Questions
Exam 30: Electromagnetic Induction66 Questions
Exam 31: Electromagnetic Fields and Waves52 Questions
Exam 32: Ac Circuits44 Questions
Exam 33: Wave Optics51 Questions
Exam 34: Ray Optics60 Questions
Exam 35: Optical Instruments52 Questions
Exam 36: Relativity49 Questions
Exam 37: The Foundations of Modern Physics7 Questions
Exam 38: Quantization45 Questions
Exam 39: Wave Functions and Uncertainty18 Questions
Exam 40: One-Dimensional Quantum Mechanics32 Questions
Exam 41: Atomic Physics38 Questions
Exam 42: Nuclear Physics64 Questions
Select questions type
A Carnot refrigerator has a coefficient of performance of 2.5.The refrigerator consumes 50 W of power.How much heat is removed from the interior of the refrigerator in 1 hour?
Free
(Multiple Choice)
5.0/5  (35)
(35)
Correct Answer:
B
Is it possible to transfer heat from a hot reservoir to a cold reservoir?
Free
(Multiple Choice)
4.8/5  (36)
(36)
Correct Answer:
B
One of the most efficient engines built so far has the following characteristics:
combustion chamber temperature = 1900°C
exhaust temperature = 430°C
7.0 × 109 cal of fuel produces 1.4 × 1010 J of work in one hour,where 1 cal = 4.19 J
(a)What is the actual efficiency of this engine?
(b)What is the Carnot efficiency of the engine?
(c)What is the power output of this engine?
Free
(Short Answer)
4.9/5  (31)
(31)
Correct Answer:
(a)48% (b)68% (c)3.9 MW
A 2.00 kg piece of lead at 40.0°C is placed in a very large quantity of water at 10.0°C,and thermal equilibrium is eventually reached.Calculate the entropy change of the lead that occurs during this process.The specific heat of lead is 130 J/(kg ∙ K).
(Multiple Choice)
4.8/5  (42)
(42)
A real (non-Carnot)heat engine,operating between heat reservoirs at temperatures of 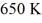 and
and 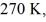 performs 4.3 kJ of net work and rejects
performs 4.3 kJ of net work and rejects 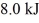 of heat in a single cycle.The thermal efficiency of this heat engine is closest to
of heat in a single cycle.The thermal efficiency of this heat engine is closest to
(Multiple Choice)
4.8/5  (37)
(37)
A refrigerator has a coefficient of performance of 1.15,and it extracts 7.95 J of heat from the cold reservoir during each cycle.
(a)How much work is done on the gas in each cycle?
(b)How much heat is exhausted into the hot reservoir in each cycle?
(Short Answer)
4.8/5  (34)
(34)
The graph in the figure shows a cycle for a heat engine for which 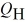 =35 J.What is the thermal efficiency of this engine?
=35 J.What is the thermal efficiency of this engine? 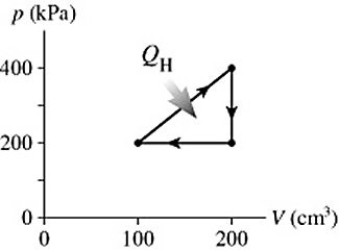
(Multiple Choice)
4.8/5  (39)
(39)
A 810-g quantity of ethanol,in the liquid state at its melting point of 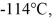 is frozen at atmospheric pressure.The heat of fusion of ethanol is 1.04 × 105 J/kg,the molecular mass is 46.1 g/mol,and the ideal gas constant is R = 8.314 J/(mol ∙ K).The change in the entropy of the ethanol as it freezes is closest to
is frozen at atmospheric pressure.The heat of fusion of ethanol is 1.04 × 105 J/kg,the molecular mass is 46.1 g/mol,and the ideal gas constant is R = 8.314 J/(mol ∙ K).The change in the entropy of the ethanol as it freezes is closest to
(Multiple Choice)
4.8/5  (32)
(32)
A Carnot engine operating between a reservoir of liquid mercury at its melting point (233 K)and a colder reservoir extracts 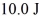 of heat from the mercury and does
of heat from the mercury and does 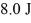 of work during each cycle.What is the temperature of the colder reservoir?
of work during each cycle.What is the temperature of the colder reservoir?
(Multiple Choice)
4.8/5  (33)
(33)
A 610-g quantity of an ideal gas undergoes a reversible isothermal compression at a temperature of 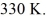 The compression reduces the volume of the gas from
The compression reduces the volume of the gas from 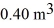 initially,to
initially,to 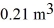 finally.The molecular mass of the gas is
finally.The molecular mass of the gas is 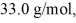 and the ideal gas constant is R = 8.314 J/(mol ∙ K).The entropy change for the gas is closest to
and the ideal gas constant is R = 8.314 J/(mol ∙ K).The entropy change for the gas is closest to
(Multiple Choice)
4.8/5  (28)
(28)
What is the maximum theoretical efficiency possible for a heat engine operating between a reservoir in which ice and water coexist,and a reservoir in which water and steam coexist? The pressure is constant at 1.0 atmosphere for both reservoirs.
(Multiple Choice)
4.8/5  (39)
(39)
A 2.00-kg block of ice at 0.00°C is dropped into a very large lake at 25.0°C and completely melts.For water,the heat of fusion is 3.35 × 105 J/kg,the heat of vaporization is 2.25 × 105 J/kg,and the specific heat is 4190 J/kg ∙ K.The net change in entropy of the system consisting of the ice and the lake due to this melting process is closest to
(Multiple Choice)
4.7/5  (31)
(31)
You want to design an ideal Carnot heat engine that wastes only 35.0% of the heat that goes into it.The lowest cold-reservoir temperature available to you is +15.0°C.If 150.0 J of work is done per cycle,the heat input per cycle is closest to
(Multiple Choice)
4.8/5  (31)
(31)
A Carnot engine is operated as a heat pump to heat a room in the winter.The heat pump delivers heat to the room at the rate of 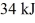 per second and maintains the room at a temperature of
per second and maintains the room at a temperature of 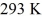 when the outside temperature is
when the outside temperature is 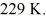 The power requirement for the heat pump under these operating conditions is closest to
The power requirement for the heat pump under these operating conditions is closest to
(Multiple Choice)
4.8/5  (39)
(39)
The temperature inside a Carnot refrigerator placed in a kitchen at 22.0°C is 2.0°C.The heat extracted from the refrigerator is 89 MJ/h.What power is needed to operate this refrigerator?
(Multiple Choice)
4.7/5  (35)
(35)
A perfect Carnot engine operates between the temperatures of 300K and 700K,drawing 60 kJ of heat from the 700K reservoir in each cycle.How much heat is dumped into the 300K reservoir in each cycle?
(Multiple Choice)
4.9/5  (38)
(38)
Is it possible to transfer heat from a cold reservoir to a hot reservoir?
(Multiple Choice)
4.8/5  (32)
(32)
A certain engine extracts 1300 J of heat from a hot temperature reservoir and discharges 700 J of heat to a cold temperature reservoir.What is the efficiency of this engine?
(Multiple Choice)
4.8/5  (29)
(29)
A Carnot refrigerator takes heat from water at 0°C and rejects heat to a room at 12°C.Suppose that 92.0 grams of water at 0°C are converted to ice at 0°C by the refrigerator.Calculate the mechanical energy that must be supplied to the refrigerator.The heat of fusion of water is 3.34 × 105 J/kg.
(Short Answer)
4.8/5  (26)
(26)
What is the change in entropy of 10.0 moles of ideal monatomic gas that reversibly undergoes the isothermal expansion shown in the figure? The ideal gas constant is R = 8.314 J/(mol ∙ K). 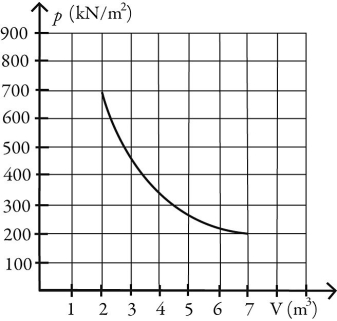
(Multiple Choice)
4.8/5  (35)
(35)
Showing 1 - 20 of 44
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)