Deck 42: Nuclear Physics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/64
Play
Full screen (f)
Deck 42: Nuclear Physics
1
The iron nucleus has the greatest binding energy of any nucleus.
False
2
A radioactive nuclide of atomic number Z emits an electron,then the daughter nuclide emits a gamma ray.What is the atomic number of the resulting nuclide after both processes?
A)Z + 1
B)Z - 1
C)Z - 2
D)Z - 3
E)Z + 2
A)Z + 1
B)Z - 1
C)Z - 2
D)Z - 3
E)Z + 2
Z + 1
3
A radioisotope has a half-life of τ at a temperature of 150 K.If its temperature is increased to 300 K,what will its half-life be?
A)4τ
B)2 τ
C)τ
D)τ/2
E)τ/4
A)4τ
B)2 τ
C)τ
D)τ/2
E)τ/4
τ
4
A certain nucleus containing 8 protons and 7 neutrons has a radius R.Which of the following values would be closest to the expected value of the radius of a nucleus having 51 protons and 69 neutrons?
A)1.85R
B)2.00R
C)2.14R
D)6.38R
E)8.00R
A)1.85R
B)2.00R
C)2.14R
D)6.38R
E)8.00R

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following statements about the atomic nucleus is correct? (There may be more than one correct choice.)
A)Large nuclei are denser than light nuclei.
B)All nuclei have nearly the same density.
C)The nucleus is held together more by the electrical force than by the gravitational force.
D)A nucleus containing 20 nucleons will have approximately twice the radius as a nucleus containing 10 nucleons.
E)As the number of nucleons increases the binding energy per nucleon always increases.
A)Large nuclei are denser than light nuclei.
B)All nuclei have nearly the same density.
C)The nucleus is held together more by the electrical force than by the gravitational force.
D)A nucleus containing 20 nucleons will have approximately twice the radius as a nucleus containing 10 nucleons.
E)As the number of nucleons increases the binding energy per nucleon always increases.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
6
The decay rate of an isotope is initially R0,but after one half-life has gone by,the rate is R0/2.At the end of the NEXT half-life,what will the decay rate be?
A)0
B)R0/16
C)R0/e
D)R0/4
E)R0/e2
A)0
B)R0/16
C)R0/e
D)R0/4
E)R0/e2

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
7
Consider two different isotopes of the same neutral element.Which statements about these isotopes are true? (There may be more than one correct choice.)
A)Both isotopes contain the same number of neutrons.
B)Both isotopes contain the same number of protons.
C)Both isotopes contain the same number of nucleons.
D)Both isotopes contain the same number of orbital electrons.
E)The sum of the protons and neutrons is the same for both isotopes.
A)Both isotopes contain the same number of neutrons.
B)Both isotopes contain the same number of protons.
C)Both isotopes contain the same number of nucleons.
D)Both isotopes contain the same number of orbital electrons.
E)The sum of the protons and neutrons is the same for both isotopes.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
8
Going from medium mass nuclei to heavy nuclei,the average binding energy per nucleon
A)decreases.
B)behaves randomly with no clear pattern.
C)does not change.
D)increases.
E)doubles.
A)decreases.
B)behaves randomly with no clear pattern.
C)does not change.
D)increases.
E)doubles.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
9
Heavier stable nuclei tend to have
A)half as many protons as neutrons.
B)the same number of neutrons and protons.
C)more neutrons than protons.
D)no clear trend in the relative number of neutrons and protons.
E)more protons than neutrons.
A)half as many protons as neutrons.
B)the same number of neutrons and protons.
C)more neutrons than protons.
D)no clear trend in the relative number of neutrons and protons.
E)more protons than neutrons.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
10
The half-life of cobalt-60 is 5.3 years,while that of strontium-90 is 28 years.Suppose you have a sample of each,such that they initially contain equal numbers of atoms of these nuclides.How will the activities (number of decays per second)of the samples compare?
A)The activity of the cobalt-60 sample will be greater.
B)The activities cannot be compared without more information.
C)The activities will be equal.
D)The activity of the strontium-90 sample will be greater.
A)The activity of the cobalt-60 sample will be greater.
B)The activities cannot be compared without more information.
C)The activities will be equal.
D)The activity of the strontium-90 sample will be greater.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
11
If a nucleus decays by β- decay to a daughter nucleus,which of the following statements about this decay are correct? (There may be more than one correct choice.)
A)The daughter nucleus has more protons than the original nucleus.
B)The daughter nucleus has more neutrons than the original nucleus.
C)The daughter nucleus has the same number of nucleons as the original nucleus.
D)The daughter nucleus has fewer protons than the original nucleus.
E)The daughter nucleus has fewer neutrons than the original nucleus.
A)The daughter nucleus has more protons than the original nucleus.
B)The daughter nucleus has more neutrons than the original nucleus.
C)The daughter nucleus has the same number of nucleons as the original nucleus.
D)The daughter nucleus has fewer protons than the original nucleus.
E)The daughter nucleus has fewer neutrons than the original nucleus.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
12
If a nucleus decays by β+ decay to a daughter nucleus,which of the following statements about this decay are correct? (There may be more than one correct choice.)
A)The daughter nucleus has more protons than the original nucleus.
B)The daughter nucleus has more neutrons than the original nucleus.
C)The daughter nucleus has the same number of nucleons as the original nucleus.
D)The daughter nucleus has fewer protons than the original nucleus.
E)The daughter nucleus has fewer neutrons than the original nucleus.
A)The daughter nucleus has more protons than the original nucleus.
B)The daughter nucleus has more neutrons than the original nucleus.
C)The daughter nucleus has the same number of nucleons as the original nucleus.
D)The daughter nucleus has fewer protons than the original nucleus.
E)The daughter nucleus has fewer neutrons than the original nucleus.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
13
For a 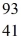 Nb atom,the number of protons,neutrons,and electrons in the atom is
Nb atom,the number of protons,neutrons,and electrons in the atom is
A)41,52,93.
B)41,52,52.
C)41,52,41.
D)41,52,0.
E)52,41,0.
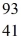 Nb atom,the number of protons,neutrons,and electrons in the atom is
Nb atom,the number of protons,neutrons,and electrons in the atom isA)41,52,93.
B)41,52,52.
C)41,52,41.
D)41,52,0.
E)52,41,0.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
14
The half-life of cobalt-60 is 5.3 years,while that of strontium-90 is 28 years.Suppose that samples of cobalt-60 and strontium-90 are such that they initially have the same activity (number of decays per second).What is true about the initial numbers of cobalt-60 and strontium-90 nuclei in these samples?
A)There are more strontium-90 than cobalt-60 nuclei.
B)There are equal numbers of cobalt-60 and strontium-90 nuclei.
C)There are more cobalt-60 than strontium-90 nuclei.
D)It is not possible to compare numbers of nuclei without knowing the masses of the samples.
A)There are more strontium-90 than cobalt-60 nuclei.
B)There are equal numbers of cobalt-60 and strontium-90 nuclei.
C)There are more cobalt-60 than strontium-90 nuclei.
D)It is not possible to compare numbers of nuclei without knowing the masses of the samples.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
15
If a nucleus decays by alpha decay to a daughter nucleus,which of the following statements about this decay are correct? (There may be more than one correct choice.)
A)The daughter nucleus has more protons than the original nucleus.
B)The daughter nucleus has more neutrons than the original nucleus.
C)The daughter nucleus has the same number of nucleons as the original nucleus.
D)The daughter nucleus has fewer protons than the original nucleus.
E)The daughter nucleus has fewer neutrons than the original nucleus.
A)The daughter nucleus has more protons than the original nucleus.
B)The daughter nucleus has more neutrons than the original nucleus.
C)The daughter nucleus has the same number of nucleons as the original nucleus.
D)The daughter nucleus has fewer protons than the original nucleus.
E)The daughter nucleus has fewer neutrons than the original nucleus.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following statements about the strong nuclear force are correct? (There may be more than one correct choice.)
A)It acts equally on protons and neutrons but not on electrons.
B)It acts equally on protons,neutrons,and electrons.
C)It has a much longer range than the electric force.
D)It keeps electrons in their orbits around the nucleus.
E)Because of its very short range,there is a limit to how large the nucleus can be.
A)It acts equally on protons and neutrons but not on electrons.
B)It acts equally on protons,neutrons,and electrons.
C)It has a much longer range than the electric force.
D)It keeps electrons in their orbits around the nucleus.
E)Because of its very short range,there is a limit to how large the nucleus can be.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following statements about β+ decay are correct? (There may be more than one correct choice.)During β+ decay
A)an orbital electron is captured by the nucleus.
B)a proton is emitted from the nucleus.
C)a neutron in the nucleus decays to a proton and an electron.
D)a proton in the nucleus decays to a positron and a neutron.
E)the atomic number Z of the isotope increases by one unit but the atomic weight A remains unchanged.
A)an orbital electron is captured by the nucleus.
B)a proton is emitted from the nucleus.
C)a neutron in the nucleus decays to a proton and an electron.
D)a proton in the nucleus decays to a positron and a neutron.
E)the atomic number Z of the isotope increases by one unit but the atomic weight A remains unchanged.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
18
If a nucleus decays by gamma decay to a daughter nucleus,which of the following statements about this decay are correct? (There may be more than one correct choice.)
A)The daughter nucleus has more protons than the original nucleus.
B)The daughter nucleus has more neutrons than the original nucleus.
C)The daughter nucleus has the same number of nucleons as the original nucleus.
D)The daughter nucleus has fewer protons than the original nucleus.
E)The daughter nucleus has fewer neutrons than the original nucleus.
A)The daughter nucleus has more protons than the original nucleus.
B)The daughter nucleus has more neutrons than the original nucleus.
C)The daughter nucleus has the same number of nucleons as the original nucleus.
D)The daughter nucleus has fewer protons than the original nucleus.
E)The daughter nucleus has fewer neutrons than the original nucleus.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
19
A radioactive isotope decays by β- emission with a half-life of 1.0 min.During the first 1.0 min,a particular sample emits 1000 β- particles.During the next 1.0 min,the number of β- particles this sample will emit will be closest to
A)250.
B)500.
C)1000.
D)1500.
E)2000.
A)250.
B)500.
C)1000.
D)1500.
E)2000.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
20
Modern nuclear bomb tests have created an extra high level of 14C in our atmosphere.Suppose that future archaeologists date samples from our era,but do not know about this testing.Will their dates be too young,too old,or still correct? If correct they are correct,why?
A)too young
B)too old
C)correct,because 14C from bomb tests is different from that produced naturally
D)correct,because modern biological materials do not gather 14C from bomb tests
A)too young
B)too old
C)correct,because 14C from bomb tests is different from that produced naturally
D)correct,because modern biological materials do not gather 14C from bomb tests

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
21
How much energy is released when 1.40 μg of 3H have decayed to 3He? The mass of 3He is 3.016029 u,the mass of 3H is 3.016049 u,and 1 u = 931.494 MeV/c2.
A)830 J
B)11,900 J
C)7970 J
D)71,700 J
E)23,900 J
A)830 J
B)11,900 J
C)7970 J
D)71,700 J
E)23,900 J

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
22
The stability of  C with respect to alpha,β+,and β- decay is to be determined.Do not consider the possibility of decay by electron capture.The following atomic masses are known:
C with respect to alpha,β+,and β- decay is to be determined.Do not consider the possibility of decay by electron capture.The following atomic masses are known: 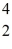 He: 4.002603 u
He: 4.002603 u 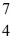 Be: 7.016928 u
Be: 7.016928 u 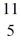 B: 11.009305 u
B: 11.009305 u 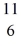 C: 11.011433 u
C: 11.011433 u 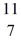 N: 11.026742 u The
N: 11.026742 u The 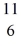 C nuclide is
C nuclide is
A)not subject to alpha,β+,or β- decay.
B)subject to alpha decay only.
C)subject to β+ decay only.
D)subject to β- decay only.
E)subject to β+ or β- decay,but not to alpha decay.
 C with respect to alpha,β+,and β- decay is to be determined.Do not consider the possibility of decay by electron capture.The following atomic masses are known:
C with respect to alpha,β+,and β- decay is to be determined.Do not consider the possibility of decay by electron capture.The following atomic masses are known: 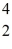 He: 4.002603 u
He: 4.002603 u 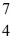 Be: 7.016928 u
Be: 7.016928 u 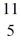 B: 11.009305 u
B: 11.009305 u 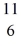 C: 11.011433 u
C: 11.011433 u 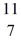 N: 11.026742 u The
N: 11.026742 u The 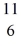 C nuclide is
C nuclide isA)not subject to alpha,β+,or β- decay.
B)subject to alpha decay only.
C)subject to β+ decay only.
D)subject to β- decay only.
E)subject to β+ or β- decay,but not to alpha decay.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
23
The neutral deuterium atom, 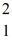 H,has a mass of 2.014102 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u.What is the binding energy of the
H,has a mass of 2.014102 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u.What is the binding energy of the 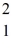 H nucleus? (1 u = 931.494 MeV/c2)
H nucleus? (1 u = 931.494 MeV/c2)
A)1.1 MeV
B)1.7 MeV
C)2.2 MeV
D)2.9 MeV
E)3.4 MeV
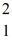 H,has a mass of 2.014102 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u.What is the binding energy of the
H,has a mass of 2.014102 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u.What is the binding energy of the 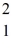 H nucleus? (1 u = 931.494 MeV/c2)
H nucleus? (1 u = 931.494 MeV/c2)A)1.1 MeV
B)1.7 MeV
C)2.2 MeV
D)2.9 MeV
E)3.4 MeV

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
24
What would be the expected radius of a nucleus having 82 protons and 125 neutrons?
A)5.2 fm
B)5.9 fm
C)6.0 fm
D)7.1 fm
E)17 fm
A)5.2 fm
B)5.9 fm
C)6.0 fm
D)7.1 fm
E)17 fm

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
25
A certain nucleus containing 8 protons and 7 neutrons has a density ρ.Which of the following values would be closest to the expected value of the density of a nucleus having 51 protons and 69 neutrons?
A)1.00 ρ
B)1.85 ρ
C)2.00 ρ
D)2.14 ρ
E)8.00 ρ
A)1.00 ρ
B)1.85 ρ
C)2.00 ρ
D)2.14 ρ
E)8.00 ρ

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
26
Two identical nuclei of mass 18 u are made to unite to make a single nucleus of mass 36 u.What is the radius of the result of this fusion?
A)4.0 fm
B)6.3 fm
C)4.5 fm
D)7.2 fm
A)4.0 fm
B)6.3 fm
C)4.5 fm
D)7.2 fm

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
27
A sphere made of a radioactive isotope initially has a mass of 6.88 kg.The half-life of this isotope is 1.34 h,and it decays by β- emission.At the end of 2.68 h,what is the mass of this sphere?
A)6.88 kg
B)3.44 kg
C)1.72 kg
D)2.53 kg
A)6.88 kg
B)3.44 kg
C)1.72 kg
D)2.53 kg

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
28
A stationary plutonium-239 nucleus decays into a uranium-235 nucleus plus an alpha particle.The energy released in the process is 5.24 MeV.Given the following mass values 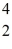 He: 4.002603 u
He: 4.002603 u 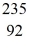 U: 235.043924 u what is the kinetic energy of the
U: 235.043924 u what is the kinetic energy of the 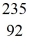 U nucleus? (1 u =931.494 MeV/c2)
U nucleus? (1 u =931.494 MeV/c2)
A)0.0829 MeV
B)0.0837 MeV
C)0.0852 MeV
D)0.0863 MeV
E)0.0877 MeV
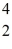 He: 4.002603 u
He: 4.002603 u 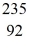 U: 235.043924 u what is the kinetic energy of the
U: 235.043924 u what is the kinetic energy of the 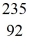 U nucleus? (1 u =931.494 MeV/c2)
U nucleus? (1 u =931.494 MeV/c2)A)0.0829 MeV
B)0.0837 MeV
C)0.0852 MeV
D)0.0863 MeV
E)0.0877 MeV

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
29
Radium-226 decays into radon-222 plus an alpha particle.How much energy is released in this process? 1 u = 931.494 MeV/c2,and the relevant mass values are 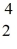 He: 4.002603 u
He: 4.002603 u  Rn: 222.017570 u
Rn: 222.017570 u  Ra: 226.025402 u
Ra: 226.025402 u
A)4.24 MeV
B)3.76 MeV
C)4.87 MeV
D)5.05 MeV
E)5.39 MeV
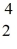 He: 4.002603 u
He: 4.002603 u 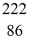 Rn: 222.017570 u
Rn: 222.017570 u 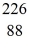 Ra: 226.025402 u
Ra: 226.025402 uA)4.24 MeV
B)3.76 MeV
C)4.87 MeV
D)5.05 MeV
E)5.39 MeV

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
30
What is the binding energy per nucleon for 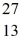 Al? The neutral
Al? The neutral 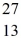 Al atom has a mass of 26.981539 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u.(1 u = 931.494 MeV/c2)
Al atom has a mass of 26.981539 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u.(1 u = 931.494 MeV/c2)
A)8.3 MeV
B)6.7 MeV
C)5.4 MeV
D)3.4 MeV
E)2.8 MeV
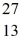 Al? The neutral
Al? The neutral 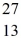 Al atom has a mass of 26.981539 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u.(1 u = 931.494 MeV/c2)
Al atom has a mass of 26.981539 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u.(1 u = 931.494 MeV/c2)A)8.3 MeV
B)6.7 MeV
C)5.4 MeV
D)3.4 MeV
E)2.8 MeV

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
31
Plutonium-239 decays into uranium-235 plus an alpha particle.The energy released in the process is 5.24 MeV.Given the following mass values 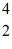 He: 4.002603 u
He: 4.002603 u 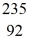 U: 235.043924 u what is the mass of
U: 235.043924 u what is the mass of 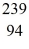 Pu in atomic mass units? (1 u = 931.494 MeV/c2)
Pu in atomic mass units? (1 u = 931.494 MeV/c2)
A)239.05215 u
B)239.02775 u
C)239.00189 u
D)238.99919 u
E)238.98884 u
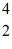 He: 4.002603 u
He: 4.002603 u 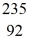 U: 235.043924 u what is the mass of
U: 235.043924 u what is the mass of 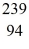 Pu in atomic mass units? (1 u = 931.494 MeV/c2)
Pu in atomic mass units? (1 u = 931.494 MeV/c2)A)239.05215 u
B)239.02775 u
C)239.00189 u
D)238.99919 u
E)238.98884 u

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
32
The stability of 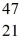 Sc with respect to alpha,β+,and β- decay is to be determined.Do not consider the possibility of decay by electron capture.The following atomic masses are known:
Sc with respect to alpha,β+,and β- decay is to be determined.Do not consider the possibility of decay by electron capture.The following atomic masses are known: 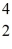 He: 4.002603 u
He: 4.002603 u 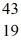 K: 42.960717 u
K: 42.960717 u 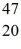 Ca: 46.954543 u
Ca: 46.954543 u 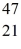 Sc 46.952409 u
Sc 46.952409 u 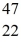 Ti: 46.951764 u The
Ti: 46.951764 u The 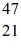 Sc nuclide is
Sc nuclide is
A)not subject to alpha,β+,or β- decay.
B)subject to alpha decay only.
C)subject to β+ decay only.
D)subject to β- decay only.
E)subject to β+ or β- decay,but not to alpha decay.
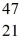 Sc with respect to alpha,β+,and β- decay is to be determined.Do not consider the possibility of decay by electron capture.The following atomic masses are known:
Sc with respect to alpha,β+,and β- decay is to be determined.Do not consider the possibility of decay by electron capture.The following atomic masses are known: 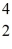 He: 4.002603 u
He: 4.002603 u 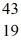 K: 42.960717 u
K: 42.960717 u 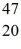 Ca: 46.954543 u
Ca: 46.954543 u 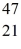 Sc 46.952409 u
Sc 46.952409 u 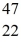 Ti: 46.951764 u The
Ti: 46.951764 u The 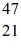 Sc nuclide is
Sc nuclide isA)not subject to alpha,β+,or β- decay.
B)subject to alpha decay only.
C)subject to β+ decay only.
D)subject to β- decay only.
E)subject to β+ or β- decay,but not to alpha decay.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
33
The set of nuclear reactions that power our sun can be summarized a 4p+ → 4He+2 + 2e+.The masses of the particles involved are 938.272 MeV/c2 (proton,p+),3727.38 MeV/c2 (alpha particle,4He+2),and 0.511 MeV/c2 (positron,e+).How much energy is released by each set of these reactions?
A)24.69 MeV
B)28.3 MeV
C)2790 MeV
D)279 MeV
A)24.69 MeV
B)28.3 MeV
C)2790 MeV
D)279 MeV

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
34
What would be the expected radius of the nucleus of 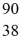 Sr?
Sr?
A)4.0 fm
B)1.2 fm
C)5.4 fm
D)0.11 pm
E)0.54 pm
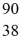 Sr?
Sr?A)4.0 fm
B)1.2 fm
C)5.4 fm
D)0.11 pm
E)0.54 pm

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
35
Scandium, 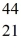 Sc,decays by emitting a positron.What is the nuclide that is the product of the decay?
Sc,decays by emitting a positron.What is the nuclide that is the product of the decay?
A)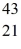 Sc
Sc
B)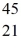 Sc
Sc
C)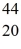 Ca
Ca
D)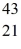 Ca
Ca
E)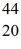 Sc
Sc
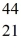 Sc,decays by emitting a positron.What is the nuclide that is the product of the decay?
Sc,decays by emitting a positron.What is the nuclide that is the product of the decay?A)
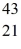 Sc
ScB)
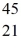 Sc
ScC)
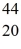 Ca
CaD)
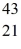 Ca
CaE)
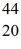 Sc
Sc
Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
36
If a nucleus had a diameter of 8.0 fm,what would be its expected mass,in atomic mass units?
A)7 u
B)296 u
C)37 u
D)64 u
E)128 u
A)7 u
B)296 u
C)37 u
D)64 u
E)128 u

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
37
A radioactive atom has 98 protons and 249 nucleons.If it undergoes alpha decay,what are the number of protons and nucleons,respectively,in the daughter nucleus?
A)100,245
B)94,247
C)96,245
D)96,247
E)100,249
A)100,245
B)94,247
C)96,245
D)96,247
E)100,249

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
38
The following masses are known: 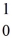 n (neutron)1.008665 u
n (neutron)1.008665 u 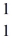 H 1.007825 u
H 1.007825 u 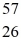 Fe 56.935399 u What is the binding energy of
Fe 56.935399 u What is the binding energy of 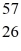 Fe,in MeV? (1 u = 1.6605 × 10-27 kg = 931.5 MeV/c2)
Fe,in MeV? (1 u = 1.6605 × 10-27 kg = 931.5 MeV/c2)
A)500 MeV
B)550 MeV
C)610 MeV
D)660 MeV
E)710 MeV
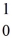 n (neutron)1.008665 u
n (neutron)1.008665 u 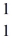 H 1.007825 u
H 1.007825 u 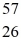 Fe 56.935399 u What is the binding energy of
Fe 56.935399 u What is the binding energy of 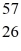 Fe,in MeV? (1 u = 1.6605 × 10-27 kg = 931.5 MeV/c2)
Fe,in MeV? (1 u = 1.6605 × 10-27 kg = 931.5 MeV/c2)A)500 MeV
B)550 MeV
C)610 MeV
D)660 MeV
E)710 MeV

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
39
The carbon in your body was formed in nuclear reactions in long-dead stars.How much energy was released when three 4He nuclei combined to make 12C? The mass of 4He is 4.002603 u,the mass of 12C is 12.0000 u,and 1 u = 931.494 MeV/c2.
A)7.274 MeV
B)3716 MeV
C)8.424 MeV
D)2.106 MeV
A)7.274 MeV
B)3716 MeV
C)8.424 MeV
D)2.106 MeV

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
40
Uranium-238 decays into thorium-234 plus an alpha particle.How much energy is released in this process? 1 u = 931.494 MeV/c2,and the relevant mass values are 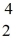 He: 4.002603 u
He: 4.002603 u 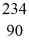 Th: 234.043583 u
Th: 234.043583 u 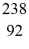 U: 238.050786 u
U: 238.050786 u
A)4.28 MeV
B)3.76 MeV
C)3.18 MeV
D)2.89 MeV
E)5.05 MeV
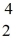 He: 4.002603 u
He: 4.002603 u 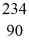 Th: 234.043583 u
Th: 234.043583 u 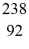 U: 238.050786 u
U: 238.050786 uA)4.28 MeV
B)3.76 MeV
C)3.18 MeV
D)2.89 MeV
E)5.05 MeV

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
41
An archaeologist finds the 14C in a sample of 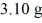 of material to be decaying at 107 counts per second.A modern 1.00-g sample of the same material decays at 151 counts per second.The half-life of 14C is 5730 y.How old is the sample?
of material to be decaying at 107 counts per second.A modern 1.00-g sample of the same material decays at 151 counts per second.The half-life of 14C is 5730 y.How old is the sample?
A)12,200 y
B)8460 y
C)25,100 y
D)12,600 y
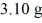 of material to be decaying at 107 counts per second.A modern 1.00-g sample of the same material decays at 151 counts per second.The half-life of 14C is 5730 y.How old is the sample?
of material to be decaying at 107 counts per second.A modern 1.00-g sample of the same material decays at 151 counts per second.The half-life of 14C is 5730 y.How old is the sample?A)12,200 y
B)8460 y
C)25,100 y
D)12,600 y

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
42
A certain substance has a half-life of 5.0 hours.How many nuclei of the substance are required to give an initial activity of 6.0 μCi? 1 Ci = 3.7 × 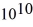 Bq.
Bq.
A)5.8 × 109
B)8.5 × 108
C)6.3 × 108
D)3.2 × 109
E)2.4 × 109
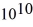 Bq.
Bq.A)5.8 × 109
B)8.5 × 108
C)6.3 × 108
D)3.2 × 109
E)2.4 × 109

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
43
An air sample is contaminated with 15O,which has a half-life of 2.03 min.One possible way to minimize its hazard is to pass it through a long pipe to allow it to decay inside the pipe until it can be safely released into the atmosphere.If the oxygen moves at a speed of 1.1 m/s in the pipe,how long must the pipe be for the sample to have decayed to 3.0% of its original activity just as it leaves the pipe?
A)680 m
B)8.0 m
C)7.0 m
D)2.0 m
A)680 m
B)8.0 m
C)7.0 m
D)2.0 m

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
44
Today,the uranium found on Earth contains 0.720% 235U (with a half-life of 0.700 billion years)and 99.28% 238U (with a half-life of 4.50 billion years).At a time 2.20 billion years ago,what percent of the uranium on Earth was 238U (assuming that no other uranium isotopes were present)?
A)95.6%
B)2.18%
C)6.29%
D)8.68%
E)4.53%
A)95.6%
B)2.18%
C)6.29%
D)8.68%
E)4.53%

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
45
A certain isotope has a half-life of 32.4 hr and a relative biological effectiveness of 3.50.A sample of this isotope initially delivers an absorbed dose of 0.240 Gy to 250 g of tissue.
(a)What was the initial equivalent dose to the tissue in rem and in sieverts?
(b)How many joules of energy did the 250-g sample initially receive from the isotope?
(a)What was the initial equivalent dose to the tissue in rem and in sieverts?
(b)How many joules of energy did the 250-g sample initially receive from the isotope?

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
46
An ancient rock is found to contain 40Ar gas,indicating that 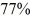 of the 40K in the rock has decayed since the rock solidified.Any argon would have boiled out of liquid rock.The half-life of 40K is 1.25 billion years.How long ago did the rock solidify?
of the 40K in the rock has decayed since the rock solidified.Any argon would have boiled out of liquid rock.The half-life of 40K is 1.25 billion years.How long ago did the rock solidify?
A)2.6 billion years
B)0.50 billion years
C)1.8 billion years
D)0.30 billion years
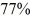 of the 40K in the rock has decayed since the rock solidified.Any argon would have boiled out of liquid rock.The half-life of 40K is 1.25 billion years.How long ago did the rock solidify?
of the 40K in the rock has decayed since the rock solidified.Any argon would have boiled out of liquid rock.The half-life of 40K is 1.25 billion years.How long ago did the rock solidify?A)2.6 billion years
B)0.50 billion years
C)1.8 billion years
D)0.30 billion years

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
47
What mass of 14C (having a half-life of 5730 years)do you need to provide a decay rate of 280.0 Bq? (1 u = 1.6605 × 10-27 kg)
A)1.70 × 10-12 kg
B)5.38 × 10-19 kg
C)3.84 × 10-20 kg
D)8.68 × 10-13 kg
A)1.70 × 10-12 kg
B)5.38 × 10-19 kg
C)3.84 × 10-20 kg
D)8.68 × 10-13 kg

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
48
The material used in certain nuclear bombs is 239Pu,which has a half-life of about 20,000 years.How long must we wait for a buried stockpile of this substance to decay to 4.0% of its original 239Pu mass?
A)93,000 y
B)64,000 y
C)45,000 y
D)800 y
A)93,000 y
B)64,000 y
C)45,000 y
D)800 y

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
49
The radioactivity due to carbon-14 measured in a piece of a wood from an ancient site was found to produce 20 counts per minute from a given sample,whereas the same amount of carbon from a piece of living wood produced 160 counts per minute.The half-life of carbon-14,a beta emitter,is 5730 y.The age of the artifact is closest to
A)5700 y.
B)12,000 y.
C)15,000 y.
D)17,000 y.
E)23,000 y.
A)5700 y.
B)12,000 y.
C)15,000 y.
D)17,000 y.
E)23,000 y.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
50
Fermium-253 has a half-life of 3.00 d.A sample of fermium contains 7.37 × 107 nuclei.How long will it take for there to be only 3.36 × 106 fermium nuclei in this sample?
A)2.75 d
B)9.80 d
C)13.4 d
D)15.7 d
E)58.6 d
A)2.75 d
B)9.80 d
C)13.4 d
D)15.7 d
E)58.6 d

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
51
The unstable isotope 234Th decays by β emission with a half-life of 24.5 days.The initial decay rate of the sample was 9.9 × 1013 Bq.(1 u = 1.6605 × 10-27 kg)
(a)What mass of 234Th was initially present?
(b)What is the decay rate after 68 days?
(a)What mass of 234Th was initially present?
(b)What is the decay rate after 68 days?

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
52
A hospital patient has been given some 131I (half-life = 8.04 d)which decays at 4.2 times the acceptable level for exposure to the general public.How long must the patient wait for the decay rate to reach the acceptable level? Assume that the material merely decays and is not excreted by the body.
A)17 d
B)12 d
C)8.0 d
D)7.2 d
A)17 d
B)12 d
C)8.0 d
D)7.2 d

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
53
Rutherfordium-261 has a half-life of 1.08 min.How long will it take for a sample of rutherfordium to lose one-third of its nuclei?
A)1.02 min
B)1.62 min
C)0.632 min
D)2.70 min
E)3.24 min
A)1.02 min
B)1.62 min
C)0.632 min
D)2.70 min
E)3.24 min

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
54
Carbon-14 has a half-life of 5730 y.A sample of wood has been recovered by an archaeologist.The sample is sent to a laboratory,where it is determined that the activity of the sample is 0.144 Bq/g.By comparing this activity with the activity of living organic matter, 0.230 Bq/g,the scientist determines how old the wood sample is,or more precisely,when the tree that the sample came from died.How old is the sample of wood?
A)3870 y
B)4250 y
C)4590 y
D)2630 y
E)2940 y
A)3870 y
B)4250 y
C)4590 y
D)2630 y
E)2940 y

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
55
Living matter has 1.3 × 10-10 % of its carbon in the form of 14C which has a half-life of 5730 y.A mammoth bone has a 300-g sample of carbon separated from it,and the sample is found to have an activity of 20 decays per second.How old is the bone?
A)15,000 y
B)10,900 y
C)11,500 y
D)7600 y
E)6400 y
A)15,000 y
B)10,900 y
C)11,500 y
D)7600 y
E)6400 y

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
56
A radioactive sample has a half-life of 10 min.What fraction of the sample is left after 40 min?
A)1/2
B)1/4
C)1/8
D)1/16
E)1/32
A)1/2
B)1/4
C)1/8
D)1/16
E)1/32

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
57
How many days are required for a radioactive sample,with a half-life of 5.7 d and an initial activity of 1.07 × 105 Bq,to decay to an activity of 100 Bq?
A)57 d
B)46 d
C)68 d
D)39 d
A)57 d
B)46 d
C)68 d
D)39 d

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
58
The stability of 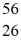 Fe with respect to alpha,β+,and β- decay is to be determined.Do not consider the possibility of decay by electron capture.The following atomic masses are known:
Fe with respect to alpha,β+,and β- decay is to be determined.Do not consider the possibility of decay by electron capture.The following atomic masses are known: 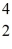 He: 4.002603 u
He: 4.002603 u 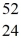 Cr: 51.944768 u
Cr: 51.944768 u 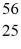 Mn: 55.938907 u
Mn: 55.938907 u 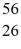 Fe: 55.934939 u
Fe: 55.934939 u 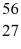 Co: 55.939841 u The
Co: 55.939841 u The 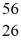 Fe nuclide is
Fe nuclide is
A)not subject to alpha,β+,or β- decay.
B)subject to alpha decay only.
C)subject to β+decay only.
D)subject to β- decay only.
E)subject to β+ or β- decay,but not to alpha decay.
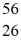 Fe with respect to alpha,β+,and β- decay is to be determined.Do not consider the possibility of decay by electron capture.The following atomic masses are known:
Fe with respect to alpha,β+,and β- decay is to be determined.Do not consider the possibility of decay by electron capture.The following atomic masses are known: 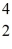 He: 4.002603 u
He: 4.002603 u 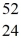 Cr: 51.944768 u
Cr: 51.944768 u 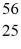 Mn: 55.938907 u
Mn: 55.938907 u 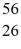 Fe: 55.934939 u
Fe: 55.934939 u 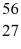 Co: 55.939841 u The
Co: 55.939841 u The 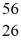 Fe nuclide is
Fe nuclide isA)not subject to alpha,β+,or β- decay.
B)subject to alpha decay only.
C)subject to β+decay only.
D)subject to β- decay only.
E)subject to β+ or β- decay,but not to alpha decay.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
59
An isotope of Tc having a half-life of 6.0 h is used in bone scans.If a certain amount of this Tc is injected into the body,how long does it take for its initial decay rate to decrease BY 99%?
A)"0.060 h"
B)"3.3 h"
C)"33 h"
D)"40 h"
E)"slightly more than a month"
A)"0.060 h"
B)"3.3 h"
C)"33 h"
D)"40 h"
E)"slightly more than a month"

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
60
In a laboratory accident a work area is contaminated with radioactive material.Health physicists monitor the area during a 30-day period and,after correcting for the background rate,obtain the data shown in the table.  The accident occurred at t = 0.They determine that it will not be safe for workers to enter the area until the radioactivity level has dropped to 133 counts per minute.Of the choices listed below,which one is the earliest time that workers could safely return?
The accident occurred at t = 0.They determine that it will not be safe for workers to enter the area until the radioactivity level has dropped to 133 counts per minute.Of the choices listed below,which one is the earliest time that workers could safely return?
A)38 days
B)44 days
C)50 days
D)32 days
E)24 days
 The accident occurred at t = 0.They determine that it will not be safe for workers to enter the area until the radioactivity level has dropped to 133 counts per minute.Of the choices listed below,which one is the earliest time that workers could safely return?
The accident occurred at t = 0.They determine that it will not be safe for workers to enter the area until the radioactivity level has dropped to 133 counts per minute.Of the choices listed below,which one is the earliest time that workers could safely return?A)38 days
B)44 days
C)50 days
D)32 days
E)24 days

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
61
A 57-kg researcher absorbs 6.3 × 108 neutrons in a workday.The energy of the neutrons is 2.6 MeV.The relative biological efficiency (RBE)for fast neutrons is 10.What is the equivalent dosage of the radiation exposure,in mrem,of this worker?
A)4.6 mrem
B)1.4 mrem
C)2.9 mrem
D)14 mrem
E)46 mrem
A)4.6 mrem
B)1.4 mrem
C)2.9 mrem
D)14 mrem
E)46 mrem

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
62
A 70-kg laboratory technician absorbs 2.9 mJ of 0.50-MeV gamma rays in a workday.How many gamma-ray photons does the technician absorb in a workday?
A)3.6 × 1010
B)3.6 × 109
C)3.6 × 108
D)1.0 × 109
E)1.0 × 108
A)3.6 × 1010
B)3.6 × 109
C)3.6 × 108
D)1.0 × 109
E)1.0 × 108

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
63
The radioactive nuclei 60Co is widely used in medical applications.It undergoes beta decay,and the total energy of the decay process is 2.82 MeV per decay event.The half-life of this nucleus is 272 days.Suppose that a patient is given a dose of 6.9 µCi of 60Co.If all of this material decayed while in the patient's body,what would be the total energy deposited there? (1 Ci = 3.70 × 1010 decays/s)
A)11 J
B)8.6 GJ
C)3.9 J
D)24 J
E)4.15 MJ
A)11 J
B)8.6 GJ
C)3.9 J
D)24 J
E)4.15 MJ

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
64
The maximum permissible workday dose for occupational exposure to radiation is 26 mrem.A 55-kg laboratory technician absorbs 3.3 mJ of 0.40-MeV gamma rays in a workday.The relative biological efficiency (RBE)for gamma rays is 1.00.What is the ratio of the equivalent dosage received by the technician to the maximum permissible equivalent dosage?
A)0.23
B)0.25
C)0.28
D)0.30
E)0.32
A)0.23
B)0.25
C)0.28
D)0.30
E)0.32

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck


