Exam 42: Nuclear Physics
Exam 1: Concepts of Motion52 Questions
Exam 2: Kinematics in One Dimension59 Questions
Exam 3: Vectors and Coordinate Systems33 Questions
Exam 4: Kinematics in Two Dimensions49 Questions
Exam 5: Force and Motion30 Questions
Exam 6: Dynamics I: Motion Along a Line46 Questions
Exam 7: Newtons Third Law43 Questions
Exam 8: Dynamics II: Motion in a Plane20 Questions
Exam 9: Work and Kinetic Energy66 Questions
Exam 10: Interactions and Potential Energy55 Questions
Exam 11: Impulse and Momentum43 Questions
Exam 12: Rotation of a Rigid Body116 Questions
Exam 13: Newtons Theory of Gravity50 Questions
Exam 14: Fluids and Elasticity72 Questions
Exam 15: Oscillations49 Questions
Exam 16: Traveling Waves51 Questions
Exam 17: Superposition51 Questions
Exam 18: A Macroscopic Description of Matter46 Questions
Exam 19: Work, heat, and the First Law of Thermodynamics96 Questions
Exam 20: The Micromacro Connection41 Questions
Exam 21: Heat Engines and Refrigerators44 Questions
Exam 22: Electric Charges and Forces26 Questions
Exam 23: The Electric Field32 Questions
Exam 24: Gausss Law41 Questions
Exam 25: The Electric Potential40 Questions
Exam 26: Potential and Field57 Questions
Exam 27: Current and Resistance32 Questions
Exam 28: Fundamentals of Circuits68 Questions
Exam 29: The Magnetic Field83 Questions
Exam 30: Electromagnetic Induction66 Questions
Exam 31: Electromagnetic Fields and Waves52 Questions
Exam 32: Ac Circuits44 Questions
Exam 33: Wave Optics51 Questions
Exam 34: Ray Optics60 Questions
Exam 35: Optical Instruments52 Questions
Exam 36: Relativity49 Questions
Exam 37: The Foundations of Modern Physics7 Questions
Exam 38: Quantization45 Questions
Exam 39: Wave Functions and Uncertainty18 Questions
Exam 40: One-Dimensional Quantum Mechanics32 Questions
Exam 41: Atomic Physics38 Questions
Exam 42: Nuclear Physics64 Questions
Select questions type
Two identical nuclei of mass 18 u are made to unite to make a single nucleus of mass 36 u.What is the radius of the result of this fusion?
Free
(Multiple Choice)
5.0/5  (30)
(30)
Correct Answer:
A
If a nucleus decays by alpha decay to a daughter nucleus,which of the following statements about this decay are correct? (There may be more than one correct choice.)
Free
(Multiple Choice)
4.8/5  (32)
(32)
Correct Answer:
D,E
A sphere made of a radioactive isotope initially has a mass of 6.88 kg.The half-life of this isotope is 1.34 h,and it decays by β- emission.At the end of 2.68 h,what is the mass of this sphere?
Free
(Multiple Choice)
4.7/5  (26)
(26)
Correct Answer:
A
Living matter has 1.3 × 10-10 % of its carbon in the form of 14C which has a half-life of 5730 y.A mammoth bone has a 300-g sample of carbon separated from it,and the sample is found to have an activity of 20 decays per second.How old is the bone?
(Multiple Choice)
4.9/5  (33)
(33)
A certain isotope has a half-life of 32.4 hr and a relative biological effectiveness of 3.50.A sample of this isotope initially delivers an absorbed dose of 0.240 Gy to 250 g of tissue.
(a)What was the initial equivalent dose to the tissue in rem and in sieverts?
(b)How many joules of energy did the 250-g sample initially receive from the isotope?
(Short Answer)
4.8/5  (29)
(29)
If a nucleus decays by β- decay to a daughter nucleus,which of the following statements about this decay are correct? (There may be more than one correct choice.)
(Multiple Choice)
4.7/5  (26)
(26)
Rutherfordium-261 has a half-life of 1.08 min.How long will it take for a sample of rutherfordium to lose one-third of its nuclei?
(Multiple Choice)
4.8/5  (28)
(28)
The radioactivity due to carbon-14 measured in a piece of a wood from an ancient site was found to produce 20 counts per minute from a given sample,whereas the same amount of carbon from a piece of living wood produced 160 counts per minute.The half-life of carbon-14,a beta emitter,is 5730 y.The age of the artifact is closest to
(Multiple Choice)
4.8/5  (34)
(34)
In a laboratory accident a work area is contaminated with radioactive material.Health physicists monitor the area during a 30-day period and,after correcting for the background rate,obtain the data shown in the table.  The accident occurred at t = 0.They determine that it will not be safe for workers to enter the area until the radioactivity level has dropped to 133 counts per minute.Of the choices listed below,which one is the earliest time that workers could safely return?
The accident occurred at t = 0.They determine that it will not be safe for workers to enter the area until the radioactivity level has dropped to 133 counts per minute.Of the choices listed below,which one is the earliest time that workers could safely return?
(Multiple Choice)
4.9/5  (33)
(33)
Today,the uranium found on Earth contains 0.720% 235U (with a half-life of 0.700 billion years)and 99.28% 238U (with a half-life of 4.50 billion years).At a time 2.20 billion years ago,what percent of the uranium on Earth was 238U (assuming that no other uranium isotopes were present)?
(Multiple Choice)
4.8/5  (42)
(42)
What would be the expected radius of a nucleus having 82 protons and 125 neutrons?
(Multiple Choice)
4.8/5  (28)
(28)
A radioisotope has a half-life of τ at a temperature of 150 K.If its temperature is increased to 300 K,what will its half-life be?
(Multiple Choice)
4.8/5  (31)
(31)
Consider two different isotopes of the same neutral element.Which statements about these isotopes are true? (There may be more than one correct choice.)
(Multiple Choice)
4.9/5  (30)
(30)
How much energy is released when 1.40 μg of 3H have decayed to 3He? The mass of 3He is 3.016029 u,the mass of 3H is 3.016049 u,and 1 u = 931.494 MeV/c2.
(Multiple Choice)
4.7/5  (36)
(36)
If a nucleus had a diameter of 8.0 fm,what would be its expected mass,in atomic mass units?
(Multiple Choice)
4.7/5  (31)
(31)
Which of the following statements about the atomic nucleus is correct? (There may be more than one correct choice.)
(Multiple Choice)
4.9/5  (39)
(39)
An ancient rock is found to contain 40Ar gas,indicating that 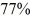 of the 40K in the rock has decayed since the rock solidified.Any argon would have boiled out of liquid rock.The half-life of 40K is 1.25 billion years.How long ago did the rock solidify?
of the 40K in the rock has decayed since the rock solidified.Any argon would have boiled out of liquid rock.The half-life of 40K is 1.25 billion years.How long ago did the rock solidify?
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following statements about the strong nuclear force are correct? (There may be more than one correct choice.)
(Multiple Choice)
4.8/5  (38)
(38)
The stability of  C with respect to alpha,β+,and β- decay is to be determined.Do not consider the possibility of decay by electron capture.The following atomic masses are known:
C with respect to alpha,β+,and β- decay is to be determined.Do not consider the possibility of decay by electron capture.The following atomic masses are known: 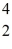 He: 4.002603 u
He: 4.002603 u 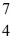 Be: 7.016928 u
Be: 7.016928 u 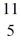 B: 11.009305 u
B: 11.009305 u 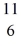 C: 11.011433 u
C: 11.011433 u 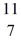 N: 11.026742 u The
N: 11.026742 u The 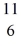 C nuclide is
C nuclide is
(Multiple Choice)
4.9/5  (33)
(33)
Showing 1 - 20 of 64
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)