Deck 4: The Three-Dimensional Structure of Proteins
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/61
Play
Full screen (f)
Deck 4: The Three-Dimensional Structure of Proteins
1
Which of the following is not an appropriate description for van der Waals interactions?
A)They involve dipole-dipole interactions.
B)Their strength depends on the distance between the two interacting atoms.
C)They are highly specific.
D)An individual van der Waals interaction does not contribute significantly to the stability of a protein.
E)They can involve hydrophobic amino acids.
A)They involve dipole-dipole interactions.
B)Their strength depends on the distance between the two interacting atoms.
C)They are highly specific.
D)An individual van der Waals interaction does not contribute significantly to the stability of a protein.
E)They can involve hydrophobic amino acids.
They are highly specific.
2
Thr and/or Leu residues tend to disrupt an helix when they occur next to each other in a protein because:
A)an amino acids like Thr is highly hydrophobic.
B)covalent interactions may occur between the Thr side chains.
C)electrostatic repulsion occurs between the Thr side chains.
D)steric hindrance occurs between the bulky Thr side chains.
E)the R group of Thr can form a hydrogen bond.
A)an amino acids like Thr is highly hydrophobic.
B)covalent interactions may occur between the Thr side chains.
C)electrostatic repulsion occurs between the Thr side chains.
D)steric hindrance occurs between the bulky Thr side chains.
E)the R group of Thr can form a hydrogen bond.
steric hindrance occurs between the bulky Thr side chains.
3
An helix would be destabilized most by:
A)an electric dipole spanning several peptide bonds throughout the helix.
B)interactions between neighboring Asp and Arg residues.
C)interactions between two adjacent hydrophobic Val residues.
D)the presence of an Arg residue near the carboxyl terminus of the helix.
E)the presence of two Lys residues near the amino terminus of the helix.
A)an electric dipole spanning several peptide bonds throughout the helix.
B)interactions between neighboring Asp and Arg residues.
C)interactions between two adjacent hydrophobic Val residues.
D)the presence of an Arg residue near the carboxyl terminus of the helix.
E)the presence of two Lys residues near the amino terminus of the helix.
the presence of two Lys residues near the amino terminus of the helix.
4
In an aqueous solution,protein conformation is determined by two major factors.One is the formation of the maximum number of hydrogen bonds.The other is the:
A)formation of the maximum number of hydrophilic interactions.
B)maximization of ionic interactions.
C)minimization of entropy by the formation of a water solvent shell around the protein.
D)placement of hydrophobic amino acid residues within the interior of the protein.
E)placement of polar amino acid residues around the exterior of the protein.
A)formation of the maximum number of hydrophilic interactions.
B)maximization of ionic interactions.
C)minimization of entropy by the formation of a water solvent shell around the protein.
D)placement of hydrophobic amino acid residues within the interior of the protein.
E)placement of polar amino acid residues around the exterior of the protein.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
5
In the helix the hydrogen bonds:
A)are roughly parallel to the axis of the helix.
B)are roughly perpendicular to the axis of the helix.
C)occur mainly between electronegative atoms of the R groups.
D)occur only between some of the amino acids of the helix.
E)occur only near the amino and carboxyl termini of the helix.
A)are roughly parallel to the axis of the helix.
B)are roughly perpendicular to the axis of the helix.
C)occur mainly between electronegative atoms of the R groups.
D)occur only between some of the amino acids of the helix.
E)occur only near the amino and carboxyl termini of the helix.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
6
In an helix,the R groups on the amino acid residues:
A)alternate between the outside and the inside of the helix.
B)are found on the outside of the helix spiral.
C)cause only right-handed helices to form.
D)generate the hydrogen bonds that form the helix.
E)stack within the interior of the helix.
A)alternate between the outside and the inside of the helix.
B)are found on the outside of the helix spiral.
C)cause only right-handed helices to form.
D)generate the hydrogen bonds that form the helix.
E)stack within the interior of the helix.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
7
The major reason that antiparallel -stranded protein structures are more stable than parallel -stranded structures is that the latter:
A)are in a slightly less extended configuration than antiparallel strands.
B)do not have as many disulfide crosslinks between adjacent strands.
C)do not stack in sheets as well as antiparallel strands.
D)have fewer lateral hydrogen bonds than antiparallel strands.
E)have weaker hydrogen bonds laterally between adjacent strands.
A)are in a slightly less extended configuration than antiparallel strands.
B)do not have as many disulfide crosslinks between adjacent strands.
C)do not stack in sheets as well as antiparallel strands.
D)have fewer lateral hydrogen bonds than antiparallel strands.
E)have weaker hydrogen bonds laterally between adjacent strands.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
8
Roughly how many amino acids are there in one turn of an helix?
A)1
B)2)8
C)3)6
D)4)2
E)10
A)1
B)2)8
C)3)6
D)4)2
E)10

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
9
The -keratin chains indicated by the diagram below have undergone one chemical step.To alter the shape of the -keratin chains-as in hair waving-what subsequent steps are required? 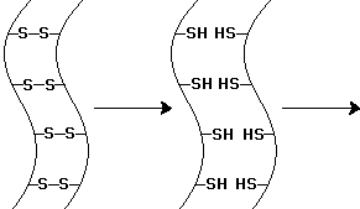
A)Chemical oxidation and then shape remodeling
B)Chemical reduction and then chemical oxidation
C)Chemical reduction and then shape remodeling
D)Shape remodeling and then chemical oxidation
E)Shape remodeling and then chemical reduction

A)Chemical oxidation and then shape remodeling
B)Chemical reduction and then chemical oxidation
C)Chemical reduction and then shape remodeling
D)Shape remodeling and then chemical oxidation
E)Shape remodeling and then chemical reduction

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following pairs of bonds within a peptide backbone show free rotation around both bonds?
A)C -C and N-C
B)C=O and N-C
C)C=O and N-C
D)N-C and C -C
E)N-C and N-C
A)C -C and N-C
B)C=O and N-C
C)C=O and N-C
D)N-C and C -C
E)N-C and N-C

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
11
Which statement about intrinsically disordered proteins is true?
A)They contain small hydrophobic cores.
B)They represent misfolded conformations of cellular proteins.
C)They have no stable three-dimensional structure and therefore have no cellular function.
D)They are responsible for proteostasis.
E)They can interact with multiple protein-binding partners and are central to protein interaction networks.
A)They contain small hydrophobic cores.
B)They represent misfolded conformations of cellular proteins.
C)They have no stable three-dimensional structure and therefore have no cellular function.
D)They are responsible for proteostasis.
E)They can interact with multiple protein-binding partners and are central to protein interaction networks.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following best represents the backbone arrangement of two peptide bonds?
A)C -N-C -C-C -N-C -C
B)C -N-C-C-N-C
C)C-N-C -C -C-N
D)C -C-N-C -C-N
E)C -C -C-N-C -C -C
A)C -N-C -C-C -N-C -C
B)C -N-C-C-N-C
C)C-N-C -C -C-N
D)C -C-N-C -C-N
E)C -C -C-N-C -C -C

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
13
The three-dimensional conformation of a protein may be strongly influenced by amino acid residues that are very far apart in sequence.This relationship is in contrast to secondary structure,where the amino acid residues are:
A)always side by side.
B)generally near each other in sequence.
C)invariably restricted to about 7 of the 20 standard amino acids.
D)often on different polypeptide strands.
E)usually near the polypeptide chain's amino terminus or carboxyl terminus.
A)always side by side.
B)generally near each other in sequence.
C)invariably restricted to about 7 of the 20 standard amino acids.
D)often on different polypeptide strands.
E)usually near the polypeptide chain's amino terminus or carboxyl terminus.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
14
In the diagram below,the plane drawn behind the peptide bond indicates the: 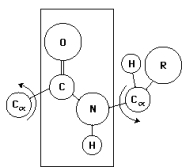
A)absence of rotation around the C-N bond because of its partial double-bond character.
B)plane of rotation around the C -N bond.
C)region of steric hindrance determined by the large C=O group.
D)region of the peptide bond that contributes to a Ramachandran plot.
E)theoretical space between -180 and +180 degrees that can be occupied by the and angles in the peptide bond.

A)absence of rotation around the C-N bond because of its partial double-bond character.
B)plane of rotation around the C -N bond.
C)region of steric hindrance determined by the large C=O group.
D)region of the peptide bond that contributes to a Ramachandran plot.
E)theoretical space between -180 and +180 degrees that can be occupied by the and angles in the peptide bond.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
15
All of the following are considered "weak" interactions in proteins except:
A)hydrogen bonds.
B)hydrophobic interactions.
C)ionic bonds.
D)peptide bonds.
E)van der Waals forces.
A)hydrogen bonds.
B)hydrophobic interactions.
C)ionic bonds.
D)peptide bonds.
E)van der Waals forces.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
16
The most important contribution to the stability of a protein's conformation appears to be the:
A)entropy increase from the decrease in ordered water molecules forming a solvent shell around it.
B)maximum entropy increase from ionic interactions between the ionized amino acids in a protein.
C)sum of free energies of formation of many weak interactions among the hundreds of amino acids in a protein.
D)sum of free energies of formation of many weak interactions between its polar amino acids and surrounding water.
E)stabilizing effect of hydrogen bonding between the carbonyl group of one peptide bond and the amino group of another.
A)entropy increase from the decrease in ordered water molecules forming a solvent shell around it.
B)maximum entropy increase from ionic interactions between the ionized amino acids in a protein.
C)sum of free energies of formation of many weak interactions among the hundreds of amino acids in a protein.
D)sum of free energies of formation of many weak interactions between its polar amino acids and surrounding water.
E)stabilizing effect of hydrogen bonding between the carbonyl group of one peptide bond and the amino group of another.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
17
A sequence of amino acids in a certain protein is found to be -Ser-Gly-Pro-Gly-.The sequence is most probably part of a(n):
A)antiparallel sheet.
B)parallel sheet.
C)( helix.)
D)( sheet.)
E)( turn.)
A)antiparallel sheet.
B)parallel sheet.
C)( helix.)
D)( sheet.)
E)( turn.)

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
18
Protein secondary structure turn are:
A)Ala and Gly.
B)hydrophobic.
C)Pro and Gly.
D)those with ionized R-groups.
E)two Cys.
A)Ala and Gly.
B)hydrophobic.
C)Pro and Gly.
D)those with ionized R-groups.
E)two Cys.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
19
Pauling and Corey's studies of the peptide bond showed that:
A)at pH 7,many different peptide bond conformations are equally probable.
B)peptide bonds are essentially planar,with no rotation about the C-N axis.
C)peptide bonds in proteins are unusual,and unlike those in small model compounds.
D)peptide bond structure is extraordinarily complex.
E)primary structure of all proteins is similar,although the secondary and tertiary structure may differ greatly.
A)at pH 7,many different peptide bond conformations are equally probable.
B)peptide bonds are essentially planar,with no rotation about the C-N axis.
C)peptide bonds in proteins are unusual,and unlike those in small model compounds.
D)peptide bond structure is extraordinarily complex.
E)primary structure of all proteins is similar,although the secondary and tertiary structure may differ greatly.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
20
A d-amino acid would interrupt an helix made of l-amino acids.Another naturally occurring hindrance to the formation of an helix is the presence of:
A)a negatively charged Arg residue.
B)a nonpolar residue near the carboxyl terminus.
C)a positively charged Lys residue.
D)a Pro residue.
E)two Ala residues side by side.
A)a negatively charged Arg residue.
B)a nonpolar residue near the carboxyl terminus.
C)a positively charged Lys residue.
D)a Pro residue.
E)two Ala residues side by side.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
21
Proteins are classified within families or superfamilies based on similarities in:
A)evolutionary origin.
B)physico-chemical properties.
C)structure and/or function.
D)subcellular location.
E)subunit structure.
A)evolutionary origin.
B)physico-chemical properties.
C)structure and/or function.
D)subcellular location.
E)subunit structure.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following is least likely to result in protein denaturation?
A)Altering net charge by changing pH
B)Changing the salt concentration
C)Disruption of weak interactions by boiling
D)Exposure to detergents
E)Mixing with organic solvents such as acetone
A)Altering net charge by changing pH
B)Changing the salt concentration
C)Disruption of weak interactions by boiling
D)Exposure to detergents
E)Mixing with organic solvents such as acetone

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following statements about oligomeric proteins is false?
A)A subunit may be similar to other proteins.
B)All subunits must be identical.
C)Many have regulatory roles.
D)Some oligomeric proteins can further associate into large fibers.
E)Some subunits may have nonprotein prosthetic groups.
A)A subunit may be similar to other proteins.
B)All subunits must be identical.
C)Many have regulatory roles.
D)Some oligomeric proteins can further associate into large fibers.
E)Some subunits may have nonprotein prosthetic groups.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
24
Any given protein is characterized by a unique amino acid sequence (primary structure)and three-dimensional (tertiary)structure.How are these related?

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
25
The structural classification of proteins (based on motifs)is based primarily on their:
A)amino-acid sequence.
B)evolutionary relationships.
C)function.
D)secondary structure content and arrangement.
E)subunit content and arrangement.
A)amino-acid sequence.
B)evolutionary relationships.
C)function.
D)secondary structure content and arrangement.
E)subunit content and arrangement.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following statements concerning the process of spontaneous folding of proteins is false?
A)It may be an essentially random process.
B)It may be defective in some human diseases.
C)It may involve a gradually decreasing range of conformational species.
D)It may involve initial formation of a highly compact state.
E)It may involve initial formation of local secondary structure.
A)It may be an essentially random process.
B)It may be defective in some human diseases.
C)It may involve a gradually decreasing range of conformational species.
D)It may involve initial formation of a highly compact state.
E)It may involve initial formation of local secondary structure.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
27
Experiments on denaturation and renaturation after the reduction and reoxidation of the -S-S- bonds in the enzyme ribonuclease (RNase)have shown that:
A)folding of denatured RNase into the native,active conformation,requires the input of energy in the form of heat.
B)native ribonuclease does not have a unique secondary and tertiary structure.
C)the completely unfolded enzyme,with all -S-S- bonds broken,is still enzymatically active.
D)the enzyme,dissolved in water,is thermodynamically stable relative to the mixture of amino acids whose residues are contained in RNase.
E)the primary sequence of RNase is sufficient to determine its specific secondary and tertiary structure.
A)folding of denatured RNase into the native,active conformation,requires the input of energy in the form of heat.
B)native ribonuclease does not have a unique secondary and tertiary structure.
C)the completely unfolded enzyme,with all -S-S- bonds broken,is still enzymatically active.
D)the enzyme,dissolved in water,is thermodynamically stable relative to the mixture of amino acids whose residues are contained in RNase.
E)the primary sequence of RNase is sufficient to determine its specific secondary and tertiary structure.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
28
A repeating structural unit in a multimeric protein is known as a(n):
A)domain.
B)motif.
C)oligomer.
D)protomer.
E)subunit.
A)domain.
B)motif.
C)oligomer.
D)protomer.
E)subunit.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
29
Proteins often have regions that can fold and function as an independent entity from the whole protein.These regions are called:
A)domains.
B)oligomers.
C)peptides.
D)sites.
E)subunits.
A)domains.
B)oligomers.
C)peptides.
D)sites.
E)subunits.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following statements concerning protein domains is true?
A)They are a form of secondary structure.
B)They are examples of structural motifs.
C)They consist of separate polypeptide chains (subunits).
D)They have been found only in prokaryotic proteins.
E)They may retain their correct shape even when separated from the rest of the protein.
A)They are a form of secondary structure.
B)They are examples of structural motifs.
C)They consist of separate polypeptide chains (subunits).
D)They have been found only in prokaryotic proteins.
E)They may retain their correct shape even when separated from the rest of the protein.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
31
When a polypeptide is in its native conformation,there are weak interactions between its R groups.However,when it is denatured there are similar interactions between the protein groups and water.What then accounts for the greater stability of the native conformation?

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
32
Draw the resonance structure of a peptide bond,and explain why there is no rotation around the C-N bond.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
33
Proteostasis is the cellular process by which:
A)proteins are synthesized.
B)proteins are folded.
C)proteins are modified.
D)proteins are degraded.
E)protein levels are maintained.
A)proteins are synthesized.
B)proteins are folded.
C)proteins are modified.
D)proteins are degraded.
E)protein levels are maintained.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following statements is false?
A)Collagen is a protein in which the polypeptides are mainly in the -helix conformation.
B)Disulfide linkages are important for keratin structure.
C)Gly residues are particularly abundant in collagen.
D)Silk fibroin is a protein in which the polypeptide is almost entirely in the conformation.
E)( -keratin is a protein in which the polypeptides are mainly in the 0-helix conformation.)
A)Collagen is a protein in which the polypeptides are mainly in the -helix conformation.
B)Disulfide linkages are important for keratin structure.
C)Gly residues are particularly abundant in collagen.
D)Silk fibroin is a protein in which the polypeptide is almost entirely in the conformation.
E)( -keratin is a protein in which the polypeptides are mainly in the 0-helix conformation.)

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
35
Name four factors (bonds or other forces)that contribute to stabilizing the native structure of a protein,and describe one condition or reagent that interferes with each type of stabilizing force.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
36
An average protein will not be denatured by:
A)a detergent such as sodium dodecyl sulfate.
B)heating to 90°C.
C)iodoacetic acid.
D)pH 10.
E)urea.
A)a detergent such as sodium dodecyl sulfate.
B)heating to 90°C.
C)iodoacetic acid.
D)pH 10.
E)urea.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
37
Pauling and Corey showed that in small peptides,six atoms associated with the peptide bond all lie in a plane.Draw a dipeptide of two amino acids in trans linkage (side-chains can be shown as -R),and indicate which six atoms are part of the planar structure of the peptide bond.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following is not known to be involved in the process of assisted folding of proteins?
A)Chaperonins
B)Disulfide interchange
C)Heat shock proteins
D)Peptide bond condensation
E)Peptide bond isomerization
A)Chaperonins
B)Disulfide interchange
C)Heat shock proteins
D)Peptide bond condensation
E)Peptide bond isomerization

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
39
Protein S will fold into its native conformation only when protein Q is also present in the solution.However,protein Q can fold into its native conformation without protein S.Protein Q,therefore,may function as a ____________ for protein S.
A)proteasome
B)molecular chaperone
C)protein precursor
D)structural motif
E)supersecondary structural unit
A)proteasome
B)molecular chaperone
C)protein precursor
D)structural motif
E)supersecondary structural unit

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
40
Kendrew's studies of the globular myoglobin structure demonstrated that:
A)"corners" between -helical regions invariably lacked proline residue.
B)highly polar or charged amino-acid residues tended to be located interiorally.
C)myoglobin was completely different from hemoglobin,as expected.
D)the structure was very compact,with virtually no internal space available for water.
E)the helix predicted by Pauling and Corey was not found in myoglobin.
A)"corners" between -helical regions invariably lacked proline residue.
B)highly polar or charged amino-acid residues tended to be located interiorally.
C)myoglobin was completely different from hemoglobin,as expected.
D)the structure was very compact,with virtually no internal space available for water.
E)the helix predicted by Pauling and Corey was not found in myoglobin.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
41
Explain (succinctly)the theoretical and/or experimental arguments in support of this statement: "The primary sequence of a protein determines its three-dimensional shape and thus its function."

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
42
Describe three of the important features of a sheet polypeptide structure.Provide one or two sentences for each feature.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
43
What is the rationale for many large proteins containing multiple copies of a polypeptide subunit?

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
44
Each of the following reagents or conditions will denature a protein.For each,describe in one or two sentences what the reagent/condition does to destroy native protein structure.
(a)urea
(b)high temperature
(c)detergent
(d)low pH
(a)urea
(b)high temperature
(c)detergent
(d)low pH

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
45
How does one determine the three-dimensional structure of a protein? Your answer should be more than the name of a technique.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
46
Once a protein has been denatured,how can it be renatured? If renaturation does not occur,what might be the explanation?

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
47
Draw the hydrogen bonding typically found between two residues in an helix.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
48
Describe three of the important features of the -helical polypeptide structure predicted by Pauling and Corey.Provide one or two sentences for each feature.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
49
Why is silk fibroin so strong,but at the same time so soft and flexible?

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
50
Explain how circular dichroism spectroscopy could be used to measure the denaturation of a protein.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
51
How can changes in pH alter the conformation of a protein?

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
52
What is typically found in the interior of a water-soluble globular protein?

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
53
In superhelical proteins,such as collagen,several polypeptide helices are intertwined.What is the function of this superhelical twisting?

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
54
Provide a brief explanation for the statement "Soluble globular proteins can be distinguished from soluble intrinsically disordered proteins on the basis of their amino acid content."

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
55
Draw a loop,and describe what is found in the interior of the loop.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
56
Describe the quaternary structure of hemoglobin.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
57
Describe a reservation about the use of x-ray crystallography in determining the three-dimensional structures of biological molecules.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
58
Explain what is meant by motifs in protein structure.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
59
Why are glycine and proline often found within a turn?

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
60
What are two mechanisms by which "chaperone" proteins assist in the correct folding of polypeptides?

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
61
What important concepts regarding protein thermal denaturation can be inferred from the egg white of a boiled egg?

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck


