Exam 4: The Three-Dimensional Structure of Proteins
Once a protein has been denatured,how can it be renatured? If renaturation does not occur,what might be the explanation?
Because a protein may be denatured through the disruption of hydrogen bonds and hydrophobic interactions by salts or organic solvents,removal of those conditions will reestablish the original aqueous environment,often permitting the protein to fold once again into its native conformation.If the protein does not renature,it may be because the denaturing treatment removed a required prosthetic group,or because the normal folding pathway requires the presence of a polypeptide chain binding protein or molecular chaperone.The normal folding pathway could also be mediated by a larger polypeptide,which is then cleaved (e.g. ,insulin).Denatured insulin would not refold easily.
In an aqueous solution,protein conformation is determined by two major factors.One is the formation of the maximum number of hydrogen bonds.The other is the:
D
How does one determine the three-dimensional structure of a protein? Your answer should be more than the name of a technique.
The protein is crystallized,and the crystal structure is determined by x-ray diffraction.The pattern of diffracted x-rays yields,by Fourier transformation,the three-dimensional distribution of electron density.By matching electron density with the known sequence of amino acids in the protein,each region of electron density is identified as a single atom.Sometimes,the three-dimensional structure of a small protein or peptide can be determined in solution by sophisticated analysis of the NMR spectrum of the polypeptide.This technique can also reveal dynamic aspects of protein structure such as conformational changes.Computer analysis of multi-dimensional NMR spectra can be used to generate a picture of the three-dimensional structure of a protein.A three dimensional structure can also be determined by cryo-electron microscopy.
When a polypeptide is in its native conformation,there are weak interactions between its R groups.However,when it is denatured there are similar interactions between the protein groups and water.What then accounts for the greater stability of the native conformation?
Which of the following best represents the backbone arrangement of two peptide bonds?
Pauling and Corey showed that in small peptides,six atoms associated with the peptide bond all lie in a plane.Draw a dipeptide of two amino acids in trans linkage (side-chains can be shown as -R),and indicate which six atoms are part of the planar structure of the peptide bond.
Which of the following statements about oligomeric proteins is false?
Kendrew's studies of the globular myoglobin structure demonstrated that:
Which of the following is least likely to result in protein denaturation?
The -keratin chains indicated by the diagram below have undergone one chemical step.To alter the shape of the -keratin chains-as in hair waving-what subsequent steps are required? 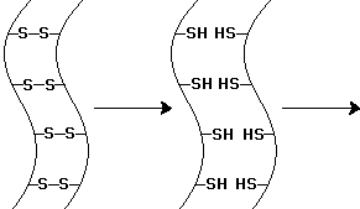
The major reason that antiparallel -stranded protein structures are more stable than parallel -stranded structures is that the latter:
What important concepts regarding protein thermal denaturation can be inferred from the egg white of a boiled egg?
Describe three of the important features of the -helical polypeptide structure predicted by Pauling and Corey.Provide one or two sentences for each feature.
Draw the hydrogen bonding typically found between two residues in an helix.
A d-amino acid would interrupt an helix made of l-amino acids.Another naturally occurring hindrance to the formation of an helix is the presence of:
Which statement about intrinsically disordered proteins is true?
What is the rationale for many large proteins containing multiple copies of a polypeptide subunit?
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)