Deck 2: Water
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/41
Play
Full screen (f)
Deck 2: Water
1
Dissolved solutes alter some physical (colligative)properties of the solvent water because they change the:
A)concentration of the water.
B)hydrogen bonding of the water.
C)ionic bonding of the water.
D)pH of the water.
E)temperature of the water.
A)concentration of the water.
B)hydrogen bonding of the water.
C)ionic bonding of the water.
D)pH of the water.
E)temperature of the water.
concentration of the water.
2
Three buffers are made by combining a 1 M solution of acetic acid with a 1 M solution of sodium acetate in the ratios shown below.

Which of these statements is true of the resulting buffers?
A)pH of buffer 1 < pH of buffer 2 < pH of buffer 3
B)pH of buffer 1 = pH of buffer 2 = pH of buffer 3
C)pH of buffer 1 > pH of buffer 2 > pH of buffer 3
D)The problem cannot be solved without knowing the value of pKa.
E)None of the above

Which of these statements is true of the resulting buffers?
A)pH of buffer 1 < pH of buffer 2 < pH of buffer 3
B)pH of buffer 1 = pH of buffer 2 = pH of buffer 3
C)pH of buffer 1 > pH of buffer 2 > pH of buffer 3
D)The problem cannot be solved without knowing the value of pKa.
E)None of the above
pH of buffer 1 > pH of buffer 2 > pH of buffer 3
3
Which of the following is true about the properties of aqueous solutions?
A)A pH change from 5.0 to 6.0 reflects an increase in the hydroxide ion concentration ([OH-])of 20%.
B)A pH change from 8.0 to 6.0 reflects a decrease in the proton concentration ([H+])by a factor of 100.
C)Charged molecules are generally insoluble in water.
D)Hydrogen bonds form readily in aqueous solutions.
E)The pH can be calculated by adding 7 to the value of the pOH.
A)A pH change from 5.0 to 6.0 reflects an increase in the hydroxide ion concentration ([OH-])of 20%.
B)A pH change from 8.0 to 6.0 reflects a decrease in the proton concentration ([H+])by a factor of 100.
C)Charged molecules are generally insoluble in water.
D)Hydrogen bonds form readily in aqueous solutions.
E)The pH can be calculated by adding 7 to the value of the pOH.
Hydrogen bonds form readily in aqueous solutions.
4
A compound is known to have a free amino group with a pKa of 8.8,and one other ionizable group with a pKa between 5 and 7.To 100 mL of a 0.2 M solution of this compound at pH 8.2 was added 40 mL of a solution of 0.2 M hydrochloric acid.The pH changed to 6.2.The pKa of the second ionizable group is:
A)The pH cannot be determined from this information.
B)5.4.
C)5.6.
D)6.0.
E)6.2.
A)The pH cannot be determined from this information.
B)5.4.
C)5.6.
D)6.0.
E)6.2.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following statements about buffers is true?
A)A buffer composed of a weak acid of pKa = 5 is stronger at pH 4 than at pH 6.
B)At pH values lower than the pKa,the salt concentration is higher than that of the acid.
C)The pH of a buffered solution remains constant no matter how much acid or base is added to the solution.
D)The strongest buffers are those composed of strong acids and strong bases.
E)When pH = pKa,the weak acid and salt concentrations in a buffer are equal.
A)A buffer composed of a weak acid of pKa = 5 is stronger at pH 4 than at pH 6.
B)At pH values lower than the pKa,the salt concentration is higher than that of the acid.
C)The pH of a buffered solution remains constant no matter how much acid or base is added to the solution.
D)The strongest buffers are those composed of strong acids and strong bases.
E)When pH = pKa,the weak acid and salt concentrations in a buffer are equal.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
6
Consider an acetate buffer,initially at the same pH as its pKa (4.76).When sodium hydroxide (NaOH)is mixed with this buffer,the:
A)pH remains constant.
B)pH rises more than if an equal amount of NaOH is added to an acetate buffer initially at pH 6.76.
C)pH rises more than if an equal amount of NaOH is added to unbuffered water at pH 4.76.
D)ratio of acetic acid to sodium acetate in the buffer falls.
E)sodium acetate formed precipitates because it is less soluble than acetic acid.
A)pH remains constant.
B)pH rises more than if an equal amount of NaOH is added to an acetate buffer initially at pH 6.76.
C)pH rises more than if an equal amount of NaOH is added to unbuffered water at pH 4.76.
D)ratio of acetic acid to sodium acetate in the buffer falls.
E)sodium acetate formed precipitates because it is less soluble than acetic acid.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
7
The aqueous solution with the lowest pH is:
A)0)01 M HCl.
B)0)1 M acetic acid (pKa = 4.86).
C)0)1 M formic acid (pKa = 3.75).
D)0)1 M HCl.
E)10-12 M NaOH.
A)0)01 M HCl.
B)0)1 M acetic acid (pKa = 4.86).
C)0)1 M formic acid (pKa = 3.75).
D)0)1 M HCl.
E)10-12 M NaOH.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
8
Hydrophobic interactions make important energetic contributions to:
A)binding of a hormone to its receptor protein.
B)enzyme-substrate interactions.
C)membrane structure.
D)three-dimensional folding of a polypeptide chain.
E)All of the above are true.
A)binding of a hormone to its receptor protein.
B)enzyme-substrate interactions.
C)membrane structure.
D)three-dimensional folding of a polypeptide chain.
E)All of the above are true.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
9
The pH of a solution of 1 M HCl is:
A)0)
B)0)1.
C)1)
D)10.
E)-1.
A)0)
B)0)1.
C)1)
D)10.
E)-1.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
10
The pH of a sample of blood is 7.4,while gastric juice is pH 1.4.The blood sample has:
A)0)189 times the [H+] as the gastric juice.
B)5)29 times lower [H+] than the gastric juice.
C)6 times lower [H+] than the gastric juice.
D)6000 times lower [H+] than the gastric juice.
E)one million times lower [H+] than the gastric juice.
A)0)189 times the [H+] as the gastric juice.
B)5)29 times lower [H+] than the gastric juice.
C)6 times lower [H+] than the gastric juice.
D)6000 times lower [H+] than the gastric juice.
E)one million times lower [H+] than the gastric juice.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
11
The Henderson-Hasselbalch equation:
A)allows the graphic determination of the molecular weight of a weak acid from its pH alone.
B)does not explain the behavior of di- or tri-basic weak acids.
C)employs the same value for pKa for all weak acids.
D)is equally useful with solutions of acetic acid and of hydrochloric acid.
E)relates the pH of a solution to the pKa and the concentrations of acid and conjugate base.
A)allows the graphic determination of the molecular weight of a weak acid from its pH alone.
B)does not explain the behavior of di- or tri-basic weak acids.
C)employs the same value for pKa for all weak acids.
D)is equally useful with solutions of acetic acid and of hydrochloric acid.
E)relates the pH of a solution to the pKa and the concentrations of acid and conjugate base.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
12
Phosphoric acid is tribasic,with pKa's of 2.14,6.86,and 12.4.The ionic form that predominates at pH 3.2 is:
A)H3PO4.
B)H2PO4-.
C)HPO42-.
D)PO43-.
E)none of the above.
A)H3PO4.
B)H2PO4-.
C)HPO42-.
D)PO43-.
E)none of the above.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
13
A compound has a pKa of 7.4.To 100 mL of a 1.0 M solution of this compound at pH 8.0 is added 30 mL of 1.0 M hydrochloric acid.The resulting solution is pH:
A)6)5.
B)6)8.
C)7)2.
D)7)4.
E)7)5.
A)6)5.
B)6)8.
C)7)2.
D)7)4.
E)7)5.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
14
Osmosis is movement of a:
A)charged solute molecule (ion)across a membrane.
B)gas molecule across a membrane.
C)nonpolar solute molecule across a membrane.
D)polar solute molecule across a membrane.
E)water molecule across a membrane.
A)charged solute molecule (ion)across a membrane.
B)gas molecule across a membrane.
C)nonpolar solute molecule across a membrane.
D)polar solute molecule across a membrane.
E)water molecule across a membrane.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
15
A true statement about hydrophobic interactions is that they:
A)are the driving force in the formation of micelles of amphipathic compounds in water.
B)do not contribute to the structure of water-soluble proteins.
C)have bonding energies of approximately 20-40 Kjoule per mole.
D)involve the ability of water to denature proteins.
E)primarily involve the effect of polar solutes on the entropy of aqueous systems.
A)are the driving force in the formation of micelles of amphipathic compounds in water.
B)do not contribute to the structure of water-soluble proteins.
C)have bonding energies of approximately 20-40 Kjoule per mole.
D)involve the ability of water to denature proteins.
E)primarily involve the effect of polar solutes on the entropy of aqueous systems.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
16
The pH of a solution of 0.1 M NaOH is:
A)0)1.
B)1)0.
C)12.8.
D)13.
E)14.
A)0)1.
B)1)0.
C)12.8.
D)13.
E)14.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
17
The aqueous solution with the highest pH is:
A)1 M HCl.
B)1 M NH3 (pKa = 9.25).
C)0)5 M NaHCO3 (pKa = 3.77).
D)0)1 M NaOH.
E)0)001 M NaOH.
A)1 M HCl.
B)1 M NH3 (pKa = 9.25).
C)0)5 M NaHCO3 (pKa = 3.77).
D)0)1 M NaOH.
E)0)001 M NaOH.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
18
A hydronium ion:
A)has the structure H3O+.
B)is a hydrated hydrogen ion.
C)is a hydrated proton.
D)is the usual form of one of the dissociation products of water in solution.
E)All of the above are true.
A)has the structure H3O+.
B)is a hydrated hydrogen ion.
C)is a hydrated proton.
D)is the usual form of one of the dissociation products of water in solution.
E)All of the above are true.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
19
A 1.0 M solution of a compound with 2 ionizable groups (pKa's = 6.2 and 9.5;100 mL total)has a pH of 6.8.If a biochemist adds 60 mL of 1.0 M HCl to this solution,the solution will change to pH:
A)5)60.
B)8)90.
C)9)13.
D)9)32.
E)The pH cannot be determined from this information.
A)5)60.
B)8)90.
C)9)13.
D)9)32.
E)The pH cannot be determined from this information.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
20
Which of these statements about hydrogen bonds is not true?
A)Hydrogen bonds account for the anomalously high boiling point of water.
B)In liquid water,the average water molecule forms hydrogen bonds with three to four other water molecules.
C)Individual hydrogen bonds are much weaker than covalent bonds.
D)Individual hydrogen bonds in liquid water exist for many seconds and sometimes for minutes.
E)The strength of a hydrogen bond depends on the linearity of the three atoms involved in the bond.
A)Hydrogen bonds account for the anomalously high boiling point of water.
B)In liquid water,the average water molecule forms hydrogen bonds with three to four other water molecules.
C)Individual hydrogen bonds are much weaker than covalent bonds.
D)Individual hydrogen bonds in liquid water exist for many seconds and sometimes for minutes.
E)The strength of a hydrogen bond depends on the linearity of the three atoms involved in the bond.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
21
Suppose you have just added 100 mL of a solution containing 0.5 mol of acetic acid per liter to 400 mL of 0.5 M NaOH.What is the final pH? (The pKa of acetic acid is 4.7. )

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
22
In which reaction below does water not participate as a reactant (rather than as a product)?
A)Conversion of an acid anhydride to two acids
B)Conversion of an ester to an acid and an alcohol
C)Conversion of ATP to ADP
D)Photosynthesis
E)Production of gaseous carbon dioxide from bicarbonate
A)Conversion of an acid anhydride to two acids
B)Conversion of an ester to an acid and an alcohol
C)Conversion of ATP to ADP
D)Photosynthesis
E)Production of gaseous carbon dioxide from bicarbonate

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following properties of water does not contribute to the fitness of the aqueous environment for living organisms?
A)Cohesion of liquid water due to hydrogen bonding
B)High heat of vaporization
C)High specific heat
D)The density of water is greater than the density of ice
E)The very low molecular weight of water
A)Cohesion of liquid water due to hydrogen bonding
B)High heat of vaporization
C)High specific heat
D)The density of water is greater than the density of ice
E)The very low molecular weight of water

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
24
For a weak acid with a pKa of 6.0,show how you would calculate the ratio of acid to salt at pH 5.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
25
(a)Briefly define "isotonic," "hypotonic," and "hypertonic" solutions.
(b)Describe what happens when a cell is placed in each of these types of solutions.
(b)Describe what happens when a cell is placed in each of these types of solutions.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
26
For each of the pairs below,circle the conjugate base.
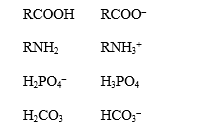


Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
27
Define pKa for a weak acid in the following two ways: (1)in relation to its acid dissociation constant,Ka,and (2)by reference to a titration curve for the weak acid.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
28
Name and briefly define five types of noncovalent interactions that occur between biological molecules.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
29
Give the general Henderson-Hasselbalch equation and sketch the plot it describes (pH against amount of NaOH added to a weak acid).On your curve,label the pKa for the weak acid and indicate the region in which the buffering capacity of the system is greatest.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
30
Draw the titration curve for a weak acid,HA,whose pKa is 3.2.Label the axes properly.Indicate with an arrow where on the curve the ratio of salt (A-)to acid (HA)is 3:1.What is the pH at this point?

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
31
In proteins,the amino acid histidine (His)plays an important role in many biological reactions.The pKa for the protonation of His to form HisH+ = 6.0.When pH = 7.0,what is the fraction of total histidine that will be in the HisH+ form?

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
32
Explain the fact that triethylammonium chloride ((CH3CH2)3N•HCl)is more soluble in water than is triethylamine ((CH3CH2)3N).

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
33
You want to maintain pH = 7.0 for an enzyme-catalyzed reaction that will produce hydrogen ions along with the desired product.At equal concentrations,which weak acid,if any,will serve as the better buffer for the reaction: Acid A,with pKa = 6.5 or Acid B,with pKa = 7.5?
A)Acid A
B)Water is as good as either of the acids available.
C)Acid B
D)Both are equally effective.
A)Acid A
B)Water is as good as either of the acids available.
C)Acid B
D)Both are equally effective.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
34
Explain with an appropriate diagram why amphipathic molecules tend to form micelles in water.What force drives micelle formation?

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
35
Explain the fact that ethanol (CH3CH2OH)is more soluble in water than is ethane (CH3CH3).

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
36
H+ + HCO3- H2CO3 CO2 + H2O
Severe diarrhea is accompanied by a loss of HCO3-.If untreated,will the condition result in acidosis or alkalosis? Use the bicarbonate buffer system given in the scheme above and Le Chatelier's Principle to explain your answer.
Severe diarrhea is accompanied by a loss of HCO3-.If untreated,will the condition result in acidosis or alkalosis? Use the bicarbonate buffer system given in the scheme above and Le Chatelier's Principle to explain your answer.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
37
Phosphoric acid (H3PO4)has three dissociable protons,with the pKa's shown below.Which form of phosphoric acid predominates in a solution at pH 4? Explain your answer.
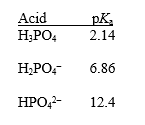


Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
38
What is the pH of a solution containing 0.2 M acetic acid (pKa = 4.7)and 0.1 M sodium acetate?

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
39
A weak acid HA,has a pKa of 5.0.If 1.0 mol of this acid and 0.1 mol of NaOH were dissolved in one liter of water,what would the final pH be?

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
40
You have just made a solution by combining 50 mL of a 0.1 M sodium acetate solution with 150 mL of 1 M acetic acid (pKa = 4.7).What is the pH of the resulting solution?

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
41
If ice were denser than water,how would that affect life on earth?

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck


