Exam 2: Water
Exam 1: The Foundations of Biochemistry56 Questions
Exam 2: Water41 Questions
Exam 3: Amino Acids, peptides, and Proteins72 Questions
Exam 4: The Three-Dimensional Structure of Proteins61 Questions
Exam 5: Protein Function45 Questions
Exam 6: Enzymes67 Questions
Exam 7: Carbohydrates and Glycobiology53 Questions
Exam 8: Nucleotides and Nucleic Acids60 Questions
Exam 9: Recombinant Dna Technology46 Questions
Exam 10: Lipids46 Questions
Exam 11: Biological Membranes and Transport60 Questions
Exam 12: Biosignaling70 Questions
Exam 13: Principles of Bioenergetics53 Questions
Exam 14: Glycolysis, gluconeogenesis, and the Pentose Phosphate Pathway79 Questions
Exam 15: Principles of Metabolic Regulation48 Questions
Exam 16: The Citric Acid Cycle58 Questions
Exam 17: Fatty Acid Catabolism50 Questions
Exam 18: Amino Acid Oxidation and the Production of Urea48 Questions
Exam 19: Oxidative Phosphorylation and Photophosphorylation78 Questions
Exam 20: Carbohydrate Biosynthesis in Plants and Bacteria47 Questions
Exam 21: Lipid Biosynthesis58 Questions
Exam 22: Biosynthesis of Amino Acids, nucleotides, and Related Molecules64 Questions
Exam 23: Integration and Hormonal Regulation of Mammalian Metabolism55 Questions
Exam 24: Genes and Chromosomes46 Questions
Exam 25: Dna Metabolism60 Questions
Exam 26: Rna Metabolism58 Questions
Exam 27: Protein Metabolism59 Questions
Exam 28: Regulation of Gene Expression57 Questions
Select questions type
The pH of a sample of blood is 7.4,while gastric juice is pH 1.4.The blood sample has:
Free
(Multiple Choice)
4.9/5  (35)
(35)
Correct Answer:
E
You have just made a solution by combining 50 mL of a 0.1 M sodium acetate solution with 150 mL of 1 M acetic acid (pKa = 4.7).What is the pH of the resulting solution?
Free
(Essay)
4.9/5  (36)
(36)
Correct Answer:
pH = pKa + log 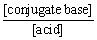 = 4.7 + log (5/150)
= 4.7 + log (5/150)
= 4.7 - 1.48 = 3.22
In proteins,the amino acid histidine (His)plays an important role in many biological reactions.The pKa for the protonation of His to form HisH+ = 6.0.When pH = 7.0,what is the fraction of total histidine that will be in the HisH+ form?
Free
(Essay)
4.8/5  (28)
(28)
Correct Answer:
Use the Henderson-Hasselbalch equation to determine the ratio of [His] to [HisH+].![Use the Henderson-Hasselbalch equation to determine the ratio of [His] to [HisH<sup>+</sup>]. To determine the fraction of the total in the HisH+ form, [His]<sub>total</sub> = [His] + [HisH+],](https://storage.examlex.com/TB1054/11ec7217_bb2f_5bfb_873d_7b55365978c8_TB1054_00.jpg)
To determine the fraction of the total in the HisH+ form, [His]total = [His] + [HisH+],![Use the Henderson-Hasselbalch equation to determine the ratio of [His] to [HisH<sup>+</sup>]. To determine the fraction of the total in the HisH+ form, [His]<sub>total</sub> = [His] + [HisH+],](https://storage.examlex.com/TB1054/11ec7217_e18e_d0ac_873d_b1e42613f4d3_TB1054_00.jpg)
Draw the titration curve for a weak acid,HA,whose pKa is 3.2.Label the axes properly.Indicate with an arrow where on the curve the ratio of salt (A-)to acid (HA)is 3:1.What is the pH at this point?
(Essay)
4.7/5  (43)
(43)
Give the general Henderson-Hasselbalch equation and sketch the plot it describes (pH against amount of NaOH added to a weak acid).On your curve,label the pKa for the weak acid and indicate the region in which the buffering capacity of the system is greatest.
(Essay)
4.7/5  (38)
(38)
Explain with an appropriate diagram why amphipathic molecules tend to form micelles in water.What force drives micelle formation?
(Essay)
4.8/5  (36)
(36)
What is the pH of a solution containing 0.2 M acetic acid (pKa = 4.7)and 0.1 M sodium acetate?
(Essay)
4.7/5  (29)
(29)
(a)Briefly define "isotonic," "hypotonic," and "hypertonic" solutions.
(b)Describe what happens when a cell is placed in each of these types of solutions.
(Essay)
4.8/5  (30)
(30)
Which of the following properties of water does not contribute to the fitness of the aqueous environment for living organisms?
(Multiple Choice)
5.0/5  (34)
(34)
Explain the fact that ethanol (CH3CH2OH)is more soluble in water than is ethane (CH3CH3).
(Essay)
4.8/5  (27)
(27)
For a weak acid with a pKa of 6.0,show how you would calculate the ratio of acid to salt at pH 5.
(Essay)
4.9/5  (34)
(34)
Suppose you have just added 100 mL of a solution containing 0.5 mol of acetic acid per liter to 400 mL of 0.5 M NaOH.What is the final pH? (The pKa of acetic acid is 4.7. )
(Essay)
4.8/5  (42)
(42)
A compound is known to have a free amino group with a pKa of 8.8,and one other ionizable group with a pKa between 5 and 7.To 100 mL of a 0.2 M solution of this compound at pH 8.2 was added 40 mL of a solution of 0.2 M hydrochloric acid.The pH changed to 6.2.The pKa of the second ionizable group is:
(Multiple Choice)
4.8/5  (25)
(25)
Which of the following is true about the properties of aqueous solutions?
(Multiple Choice)
4.9/5  (31)
(31)
H+ + HCO3- H2CO3 CO2 + H2O
Severe diarrhea is accompanied by a loss of HCO3-.If untreated,will the condition result in acidosis or alkalosis? Use the bicarbonate buffer system given in the scheme above and Le Chatelier's Principle to explain your answer.
(Essay)
4.8/5  (32)
(32)
Hydrophobic interactions make important energetic contributions to:
(Multiple Choice)
4.8/5  (34)
(34)
Showing 1 - 20 of 41
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)