Deck 9: Liquids and Solids
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/52
Play
Full screen (f)
Deck 9: Liquids and Solids
1
Which of the following statements is true for an ideal gas?
A) A plot of ln P vsersus 1/T (in Kelvin)yields a straight line with a slope equal to −ΔHvap.
B) A plot of ln P versus T (in Kelvin)yields a straight line with a slope equal to ΔHvap.
C) A plot of ln P versus 1/T (in Kelvin)yields a straight line with a slope equal to −ΔHvap/R.
D) A plot of P versus 1/T (in Kelvin)yields a straight line with a slope equal to 1/−ΔHvap.
E) A plot of P versus T (in Kelvin)yields a straight line with a slope equal to −ΔHvap/RT.
A) A plot of ln P vsersus 1/T (in Kelvin)yields a straight line with a slope equal to −ΔHvap.
B) A plot of ln P versus T (in Kelvin)yields a straight line with a slope equal to ΔHvap.
C) A plot of ln P versus 1/T (in Kelvin)yields a straight line with a slope equal to −ΔHvap/R.
D) A plot of P versus 1/T (in Kelvin)yields a straight line with a slope equal to 1/−ΔHvap.
E) A plot of P versus T (in Kelvin)yields a straight line with a slope equal to −ΔHvap/RT.
A plot of ln P versus 1/T (in Kelvin)yields a straight line with a slope equal to −ΔHvap/R.
2
On the phase diagram below,which point corresponds to conditions where solid,liquid,and gas phases all exist? 
A) B
B) C
C) D
D) E
E) G

A) B
B) C
C) D
D) E
E) G
D
3
If a pure substance begins at point A on the phase diagram below and the pressure on the substance is reduced at constant temperature until point B is reached,what process occurs? 
A) fusion
B) vaporization
C) condensation
D) sublimation
E) none of the above

A) fusion
B) vaporization
C) condensation
D) sublimation
E) none of the above
sublimation
4
Mount Everest rises to a height of 29,035 ft (8.850 × 103 m)above sea level.At this height,the atmospheric pressure is 230 mm Hg.At what temperature does water boil at the summit of Mount Everest? (ΔHvap for H2O = 40.7 kJ/mole,R = 8.31 J/K⋅mol)
A) −17°C
B) 31°C
C) 48°C
D) 57°C
E) 69°C
A) −17°C
B) 31°C
C) 48°C
D) 57°C
E) 69°C

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
5
Carbon tetrachloride,an organic solvent,has a molar heat of vaporization of 29.82 kJ/mol.If CCl4 has a normal boiling point of 3.50 × 102 K,what is its vapor pressure at 273 K? (R = 8.31 J/mol⋅K)
A) 19.3 mm Hg
B) 42.2 mm Hg
C) 59.3 mm Hg
D) 108 mm Hg
E) 359 mm Hg
A) 19.3 mm Hg
B) 42.2 mm Hg
C) 59.3 mm Hg
D) 108 mm Hg
E) 359 mm Hg

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
6
Xenon has a molar heat of vaporization of 12.6 kJ/mol and a vapor pressure of 1.00 atm at -108.0°C.What is the vapor pressure of xenon at -148.0°C? (R = 8.31 J/K⋅mol)
A) 0.053 atm
B) 0.73 atm
C) 0.93 atm
D) 0.99 atm
E) 19 atm
A) 0.053 atm
B) 0.73 atm
C) 0.93 atm
D) 0.99 atm
E) 19 atm

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
7
All of the following statements are incorrect EXCEPT
A) gas,liquid and solid phases of a substance exist simultaneously at equilibrium at the critical pressure and temperature.
B) above the critical pressure,only the solid phase of a pure substance can exist.
C) the liquid phase of a pure substance cannot exist above its critical temperature.
D) the critical temperature and pressure cannot be produced simultaneously.
E) above the critical temperature and pressure,only the liquid phase of a substance exists.
A) gas,liquid and solid phases of a substance exist simultaneously at equilibrium at the critical pressure and temperature.
B) above the critical pressure,only the solid phase of a pure substance can exist.
C) the liquid phase of a pure substance cannot exist above its critical temperature.
D) the critical temperature and pressure cannot be produced simultaneously.
E) above the critical temperature and pressure,only the liquid phase of a substance exists.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
8
Methyl alcohol,CH3OH,has a vapor pressure of 203 mm Hg at 35°C.If 5.00 g CH3OH is sealed in a 10.0 L flask,what mass will remain in the liquid phase when equilibrium is established at 35°C? Assume any liquid remaining in the flask has a negligible volume.(760 mm Hg = 1 atm,R = 0.0821 L⋅atm/mol⋅K)
A) 0.00 g
B) 1.62 g
C) 2.08 g
D) 2.27 g
E) 4.69 g
A) 0.00 g
B) 1.62 g
C) 2.08 g
D) 2.27 g
E) 4.69 g

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
9
What process occurs when a substance is at point C on the phase diagram below,and the pressure is decreased (under constant temperature)until the substance is at point E? 
A) condensation
B) vaporization
C) sublimation
D) melting
E) freezing

A) condensation
B) vaporization
C) sublimation
D) melting
E) freezing

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
10
Freon-113,C2Cl3F3,has an enthalpy of vaporization of 27.04 kJ/mol and a normal boiling point of 48°C.At what temperature is the vapor pressure of Freon-113 equal to 76.0 mm Hg? (R = 8.31 J/K⋅mol)
A) −53.1°C
B) −23.8°C
C) −11.4°C
D) 1.44°C
E) 7.12°C
A) −53.1°C
B) −23.8°C
C) −11.4°C
D) 1.44°C
E) 7.12°C

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
11
The normal boiling point of a liquid is
A) 373 K.
B) the temperature at which a liquid's vapor pressure equals 1 atm.
C) the pressure at which the liquid boils at 373 K.
D) dependent upon the volume of the liquid.
E) dependent upon the surface area of the liquid..
A) 373 K.
B) the temperature at which a liquid's vapor pressure equals 1 atm.
C) the pressure at which the liquid boils at 373 K.
D) dependent upon the volume of the liquid.
E) dependent upon the surface area of the liquid..

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
12
Sulfur dioxide has a vapor pressure of 462.7 mm Hg at -21.0°C and a vapor pressure of 140.5 mm Hg at -44.0°C.What is the molar heat of vaporization of sulfur dioxide? (R = 8.31 J/K⋅mol)
A) 0.398 kJ/mol
B) 6.33 kJ/mol
C) 14.0 kJ/mol
D) 24.9 kJ/mol
E) 39.8 kJ/mol
A) 0.398 kJ/mol
B) 6.33 kJ/mol
C) 14.0 kJ/mol
D) 24.9 kJ/mol
E) 39.8 kJ/mol

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following equations is a correct form of the Clausius-Clapeyron equation?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
14
Sulfur dioxide has an enthalpy of vaporization of 24.9 kJ/mol.At 205 K,SO2 has a vapor pressure of 30.3 mm Hg.What is the normal boiling point temperature of SO2? (R = 8.31 J/K⋅mol)
A) 263 K
B) 278 K
C) 291 K
D) 308 K
E) 345 K
A) 263 K
B) 278 K
C) 291 K
D) 308 K
E) 345 K

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
15
Ethanol has a molar heat of vaporization of 42.3 kJ/mol.The compound has a vapor pressure of 1.00 atm at 78.3°C.At what temperature is the vapor pressure equal to 0.800 atm? (R = 8.31 J/K⋅mol)
A) -83.8°C
B) -24.4°C
C) 62.6°C
D) 73.0°C
E) 78.0°C
A) -83.8°C
B) -24.4°C
C) 62.6°C
D) 73.0°C
E) 78.0°C

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
16
At 75.0 °C,water has an equilibrium vapor pressure of 289.1 mm Hg.If 4.22 g H2O is sealed in an evacuated 5.00 L flask and heated to 75.0 °C,what mass of H2O will be found in the gas phase when liquid-vapor equilibrium is established? Assume any liquid remaining in the flask has a negligible volume.(760 mm Hg = 1 atm,R = 0.0821 L⋅atm/mol⋅K)
A) 0.240 g
B) 1.20 g
C) 2.64 g
D) 3.02 g
E) 4.22 g
A) 0.240 g
B) 1.20 g
C) 2.64 g
D) 3.02 g
E) 4.22 g

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
17
Naphthalene,a substance present in some mothballs,has a molar heat of vaporization of 49.4 kJ/mol.If the vapor pressure of naphthalene is 0.300 mm Hg at 298 K,what is the temperature at which the vapor pressure is 7.60 × 102 mm Hg? (R = 8.31 J/K⋅mol)
A) 424 K
B) 491 K
C) 501 K
D) 521 K
E) 611 K
A) 424 K
B) 491 K
C) 501 K
D) 521 K
E) 611 K

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
18
On the phase diagram below,which point corresponds to conditions where both liquid and gas phases exist? 
A) A
B) B
C) C
D) E
E) F

A) A
B) B
C) C
D) E
E) F

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
19
Water has an equilibrium vapor pressure of 23.8 mm Hg at 25°C.What mass of water vapor is present,at equilibrium,in a room with dimensions of 7.0 m × 8.0 m × 2.7 m? (760 mm Hg = 1 atm,R = 0.0821 L⋅atm/mol⋅K)
A) 0.0050 kg
B) 0.029 kg
C) 0.28 kg
D) 3.5 kg
E) 5.0 kg
A) 0.0050 kg
B) 0.029 kg
C) 0.28 kg
D) 3.5 kg
E) 5.0 kg

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
20
On the phase diagram below,which point corresponds to conditions where only the solid phase exists? 
A) A
B) B
C) C
D) D
E) G

A) A
B) B
C) C
D) D
E) G

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following nonpolar molecules has the highest boiling point?
A) F2
B) C2H4
C) F2..
D) O2
E) CS2
A) F2
B) C2H4
C) F2..
D) O2
E) CS2

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
22
All of the following statements concerning dispersion forces are correct EXCEPT
A) the strength of dispersion forces depends on the number of electrons in a molecule.
B) dispersion forces are the primary attractive forces in metal-metal bonding.
C) dispersion forces involve the attraction between induced dipoles.
D) dispersion forces are the primary attractive force in molecular solids consisting of nonpolar molecules.
E) dispersion forces exist in all molecular solids.
A) the strength of dispersion forces depends on the number of electrons in a molecule.
B) dispersion forces are the primary attractive forces in metal-metal bonding.
C) dispersion forces involve the attraction between induced dipoles.
D) dispersion forces are the primary attractive force in molecular solids consisting of nonpolar molecules.
E) dispersion forces exist in all molecular solids.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following substances will exhibit dipole forces?
A) SO3
B) H2S
C) CH4
D) SF6
E) N2
A) SO3
B) H2S
C) CH4
D) SF6
E) N2

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
24
All of the following substances will exhibit dipole forces EXCEPT
A) SO3.
B) SF2.
C) H2Se.
D) NO2.
E) NO.
A) SO3.
B) SF2.
C) H2Se.
D) NO2.
E) NO.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
25
Arrange N2,O2,He,and Cl2 in order from lowest to highest melting point.
A) O2 < N2 < Cl2 < He
B) N2 < O2 < He < Cl2
C) He < Cl2 < N2 < O2
D) He < O2 < N2 < Cl2
E) He < N2 < O2 < Cl2
A) O2 < N2 < Cl2 < He
B) N2 < O2 < He < Cl2
C) He < Cl2 < N2 < O2
D) He < O2 < N2 < Cl2
E) He < N2 < O2 < Cl2

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following pure substances will have hydrogen bonds? (Lone electron pairs have been omitted from these structures. ) 
A) Acetone
B) Dimethyl ether
C) Methanol
D) Acetone and methanol
E) Dimethyl ether and methanol

A) Acetone
B) Dimethyl ether
C) Methanol
D) Acetone and methanol
E) Dimethyl ether and methanol

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
27
All of the following statements concerning hydrogen bonding are correct EXCEPT
A) water is less dense in the solid phase than the liquid phase due to hydrogen bonding.
B) hydrogen bonding occurs in molecules with N−H,O−H,and F−H bonds.
C) hydrogen bonding in water results in lower than expected melting points.
D) the unusually high boiling points of water,ammonia,and hydrogen fluoride are the result of hydrogen bonding.
E) hydrogen bonding is an unusually strong dipole force.
A) water is less dense in the solid phase than the liquid phase due to hydrogen bonding.
B) hydrogen bonding occurs in molecules with N−H,O−H,and F−H bonds.
C) hydrogen bonding in water results in lower than expected melting points.
D) the unusually high boiling points of water,ammonia,and hydrogen fluoride are the result of hydrogen bonding.
E) hydrogen bonding is an unusually strong dipole force.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
28
Arrange Cl2,ICl,and Br2 in order from lowest to highest melting point.
A) Br2 < ICl < Cl2
B) Br2 < Cl2 < ICl
C) Cl2 < ICl < Br2
D) Cl2 < Br2 < ICl
E) ICl < Br2 < Cl2
A) Br2 < ICl < Cl2
B) Br2 < Cl2 < ICl
C) Cl2 < ICl < Br2
D) Cl2 < Br2 < ICl
E) ICl < Br2 < Cl2

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
29
What is the correct method for determining the number of atoms in a face-centered cubic cell?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
30
What is a correct method for determining the number of atoms in a body-centered cubic cell?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
31
What is the dominant intermolecular force in HF(l)?
A) Dispersion forces
B) Hydrogen bonding
C) Ionic bonding
D) Dipole forces
E) Induced dipole forces
A) Dispersion forces
B) Hydrogen bonding
C) Ionic bonding
D) Dipole forces
E) Induced dipole forces

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
32
Arrange the noble gases in order from weakest to strongest interatomic forces.
A) Kr < Ar < Ne < He
B) Ne < HeC) He < Ar < Ne < Kr
D) He < Ne < Ar < Kr
E) Ar < Kr < He < Ne
A) Kr < Ar < Ne < He
B) Ne < He
D) He < Ne < Ar < Kr
E) Ar < Kr < He < Ne

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
33
All of the following statements are correct EXCEPT
A) network covalent solids are usually good electrical conductors.
B) network covalent solids often have high melting points.
C) network covalent solids are insoluble in common solvents.
D) ionic solids typically have high melting points.
E) ionic solids are often hard and brittle.
A) network covalent solids are usually good electrical conductors.
B) network covalent solids often have high melting points.
C) network covalent solids are insoluble in common solvents.
D) ionic solids typically have high melting points.
E) ionic solids are often hard and brittle.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
34
How many unit cells share an atom which is located at a corner (or lattice point)of a simple cubic unit cell?
A) 1
B) 2
C) 4
D) 6
E) 8
A) 1
B) 2
C) 4
D) 6
E) 8

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
35
Which one of the following molecules has the lowest boiling point?
A) CH4
B) CHCl3
C) CH2Cl2
D) CH3Cl
E) CCl4
A) CH4
B) CHCl3
C) CH2Cl2
D) CH3Cl
E) CCl4

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
36
Arrange NH3,CH4,and PH3 in order from lowest to highest boiling points.
A) CH4 < PH3 < NH3
B) CH4 < NH3 < PH3
C) PH3 < NH3 < CH4
D) NH3 < CH4 < PH3
E) PH3 < CH4 < NH3
A) CH4 < PH3 < NH3
B) CH4 < NH3 < PH3
C) PH3 < NH3 < CH4
D) NH3 < CH4 < PH3
E) PH3 < CH4 < NH3

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
37
Which one of the following molecules will exhibit dipole forces as a pure liquid or solid?
A) CS2
B) C2H2
C) CCl4
D) Br2
E) PH3
A) CS2
B) C2H2
C) CCl4
D) Br2
E) PH3

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
38
Elemental phosphorus is a molecular solid consisting of P4 molecules.It melts at 44°C.What is the principal force present in P4(s)?
A) Dipole forces
B) Hydrogen bonding
C) Dispersion forces
D) Metallic bonding
E) Ionic bonding
A) Dipole forces
B) Hydrogen bonding
C) Dispersion forces
D) Metallic bonding
E) Ionic bonding

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
39
In the phase diagram below,which phase is most dense? 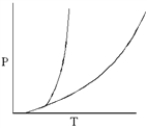
A) solid
B) liquid
C) gas
D) supercritical fluid
E) none of the above

A) solid
B) liquid
C) gas
D) supercritical fluid
E) none of the above

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
40
In which of the following pure solids is it necessary to break covalent bonds in order to make a liquid or gas?
A) SO2
B) NaCl
C) H2SO4
D) I2
E) SiO2
A) SO2
B) NaCl
C) H2SO4
D) I2
E) SiO2

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
41
For a simple cubic unit cell,what percentage of the space in the cell is occupied by the atoms at the corners of the cell?
A) 47.6%
B) 52.4%
C) 57.4%
D) 62.3%
E) 71.2%
A) 47.6%
B) 52.4%
C) 57.4%
D) 62.3%
E) 71.2%

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
42
What type of solid is conductive when melted but not as a solid?
A) Metallic
B) Ionic
C) Molecular
D) Crystalline
E) Covalent
A) Metallic
B) Ionic
C) Molecular
D) Crystalline
E) Covalent

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
43
Arrange the three common unit cells in order from least dense to most dense packing.
A) simple cubic < body-centered cubic < face-centered cubic
B) simple cubic < face-centered cubic < body-centered cubic
C) body-centered cubic < face-centered cubic < simple cubic
D) body-centered cubic < simple cubic < face-centered cubic
E) face-centered cubic < body-centered cubic < simple cubic
A) simple cubic < body-centered cubic < face-centered cubic
B) simple cubic < face-centered cubic < body-centered cubic
C) body-centered cubic < face-centered cubic < simple cubic
D) body-centered cubic < simple cubic < face-centered cubic
E) face-centered cubic < body-centered cubic < simple cubic

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
44
Iron packs in a body-centered cubic structure.If an iron atom has a radius of 126 pm,what is the distance between lattice points at two opposite corners of the unit cell? (Note: a line drawn between the points would go through the center of the unit cell)
A)
B) 2(126 pm)= 252 pm
C)
D) 4(126 pm)= 504 pm
E)
A)
B) 2(126 pm)= 252 pm
C)
D) 4(126 pm)= 504 pm
E)

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
45
Dorothy Crowfoot Hodgkin was famous for what?
A) The discovery of x-rays
B) The invention of x-ray diffraction
C) The discovery of supercritical fluids
D) The determination of the structure of insulin
E) All of the above
A) The discovery of x-rays
B) The invention of x-ray diffraction
C) The discovery of supercritical fluids
D) The determination of the structure of insulin
E) All of the above

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
46
Niobium crystallizes in a body-centered cubic unit cell.If the radius of a niobium atom is 0.145 nm,what is the length of an edge of the unit cell?
A) 0.251 nm
B) 0.290 nm
C) 0.335 nm
D) 0.410 nm
E) 0.502 nm
A) 0.251 nm
B) 0.290 nm
C) 0.335 nm
D) 0.410 nm
E) 0.502 nm

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
47
What is the distance,in atomic radii,along any edge of a face-centered unit cell?
A)
B) 2 × r
C) 4 × r
D)
E) r
A)
B) 2 × r
C) 4 × r
D)
E) r

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
48
Rhodium (atomic mass 102.9 g/mol)crystallizes in a face-centered cubic unit cell.In addition,rhodium has an atomic radius of 135 pm.What is the density (in g/cm3)of rhodium?
A) 1.53 g/cm3
B) 6.14 g/cm3
C) 17.4 g/cm3
D) 12.3 g/cm3
E) 27.8 g/cm3
A) 1.53 g/cm3
B) 6.14 g/cm3
C) 17.4 g/cm3
D) 12.3 g/cm3
E) 27.8 g/cm3

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
49
What type of solid is generally soluble in nonpolar solvents?
A) Metallic
B) Ionic
C) Molecular
D) Crystalline
E) Covalent
A) Metallic
B) Ionic
C) Molecular
D) Crystalline
E) Covalent

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
50
Potassium crystallizes in a body-centered cubic unit cell.If the length of an edge of the unit cell is 524 pm,what is the atomic radius (in pm)of a potassium atom?
A) 151 pm
B) 185 pm
C) 227 pm
D) 262 pm
E) 371 pm
A) 151 pm
B) 185 pm
C) 227 pm
D) 262 pm
E) 371 pm

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
51
Nickel has a face-centered cubic cell,and its density is 8.90 g/cm3.What is the radius (in pm)of a nickel atom? (The molar mass of nickel is 58.69 g/mol)
A) 62.3 pm
B) 88.1 pm
C) 125 pm
D) 249 pm
E) 535 pm
A) 62.3 pm
B) 88.1 pm
C) 125 pm
D) 249 pm
E) 535 pm

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
52
What type of solid is malleable?
A) Metallic
B) Ionic
C) Molecular
D) Crystalline
E) Covalent
A) Metallic
B) Ionic
C) Molecular
D) Crystalline
E) Covalent

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck


